��Ŀ����
�����ѱ���Ϊ������֮������ġ��������������������ϼ�Ϊ+4�����ǿռ似����������������ҽ���ϲ���ȱ�ٵIJ��ϡ�
��ҵ������������Ҫ�ɷ�FeTiO3���Ʊ������ѵ�һ�ֹ�����������ͼ�����ֲ�����ȥ����
��1������ٷ�Ӧ�Ļ�ѧ����ʽΪ��2FeTiO3 + 6C + 7Cl2 2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
��2������ڷ����TiCl4�ķ�����������TiCl4��FeCl3�� ������ �IJ�ͬ��
��3������ܷ�Ӧ�Ļ�ѧ����ʽΪ______________ __________���÷�Ӧ������н��е�������_________ _____________________��
��4����ɫ��ѧ�ᳫ����ѭ�������������У�������ѭ�������ʳ�Cl2��Mg�⣬����___��
��ҵ������������Ҫ�ɷ�FeTiO3���Ʊ������ѵ�һ�ֹ�����������ͼ�����ֲ�����ȥ����

��1������ٷ�Ӧ�Ļ�ѧ����ʽΪ��2FeTiO3 + 6C + 7Cl2
 2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ���2������ڷ����TiCl4�ķ�����������TiCl4��FeCl3�� ������ �IJ�ͬ��
��3������ܷ�Ӧ�Ļ�ѧ����ʽΪ______________ __________���÷�Ӧ������н��е�������_________ _____________________��
��4����ɫ��ѧ�ᳫ����ѭ�������������У�������ѭ�������ʳ�Cl2��Mg�⣬����___��
��1��FeTiO3��C�� 7 ��2���е�
��3��2Mg + TiCl4 2MgCl2 + Ti�� ��ֹMg��Ti������ ��4��MgCl2
2MgCl2 + Ti�� ��ֹMg��Ti������ ��4��MgCl2
��3��2Mg + TiCl4
 2MgCl2 + Ti�� ��ֹMg��Ti������ ��4��MgCl2
2MgCl2 + Ti�� ��ֹMg��Ti������ ��4��MgCl2�����������1�����ݷ�Ӧ�ķ���ʽ��֪��̼Ԫ�صĻ��ϼۺ�FeTiO3��Fe�Ļ��ϼ�Ҳ�����ߵ��ģ����Ի�ԭ����FeTiO3��C�����������������õ�2�����ӣ����Ը��ݷ���ʽ��֪ÿ����1mol TiCl4ת��7mol���ӡ�
��2��TiCl4��FeCl3�ķе㲻ͬ�����Բ���ڷ����TiCl4����������ķ������з��롣
��3��þ�����Ȼ����ڸ��µ������������Ȼ�þ�ͽ����ѣ���Ӧ�Ļ�ѧ����ʽ��2Mg + TiCl4
 2MgCl2 + Ti�������ѧ�����ȶ������Է�ֹMg��Ti��������
2MgCl2 + Ti�������ѧ�����ȶ������Է�ֹMg��Ti����������4����ɫ��ѧ�ᳫ����ѭ���������������У�������ѭ�������ʳ�Cl2��Mg���⣬�����Ȼ�þ�ȡ�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����������Ҫ��ȷ�������ʵ��Ʊ���һ�¼������ͣ�һ����ֻ������Ŀ������ԭ������ȡ��һ�����Լ�ѡȡԭ�����Ʊ������ʣ����е��Ǹ���һ����ҩƷ��������ѡ�ȡ���������Ŀ������ԭ������ȡ����˽�����ʵ����ʣ����Ӧ�ã��𰸾ͻ�ӭ�ж��⡣

��ϰ��ϵ�д�
�����Ŀ


 8Cu+4FeO+2Fe2O3+16SO2�������йظ÷�Ӧ��˵����ȷ���� ___________________ ������ĸ����
8Cu+4FeO+2Fe2O3+16SO2�������йظ÷�Ӧ��˵����ȷ���� ___________________ ������ĸ���� MnCl2 + 2H2O + Cl2��
MnCl2 + 2H2O + Cl2�� ������˵������ȷ����( )
������˵������ȷ����( ) ��������
��������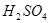 �Ȳ����������ֲ��ǻ�ԭ��
�Ȳ����������ֲ��ǻ�ԭ��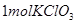 �μӷ�Ӧʱ��
�μӷ�Ӧʱ�� ����ת��
����ת��