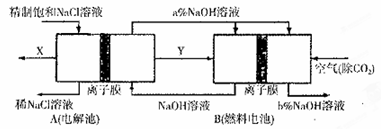
��Ŀ����
��12�֣���ͼ�漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�C��D��E��FΪ���ʣ�����������Ϊ����������������Ϊ���������Һ�����Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��
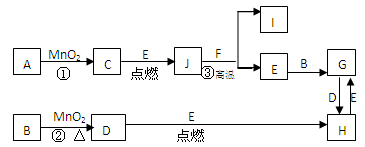
��1��д����ѧʽ��A ��E ��
��2��ָ��MnO2����ط�Ӧ�е����ã���Ӧ������ ������Ӧ������ ����
��3����Ӧ�۵Ļ�ѧ����ʽΪ ��
��4��G������B��NaNO3��ϡ��Һ��Ϸ�Ӧ����H����д���˷�Ӧ�����ӷ���ʽ ��

��1��д����ѧʽ��A ��E ��
��2��ָ��MnO2����ط�Ӧ�е����ã���Ӧ������ ������Ӧ������ ����
��3����Ӧ�۵Ļ�ѧ����ʽΪ ��
��4��G������B��NaNO3��ϡ��Һ��Ϸ�Ӧ����H����д���˷�Ӧ�����ӷ���ʽ ��
(2��/�գ���12��)
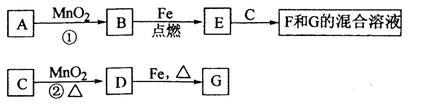
��1��H2O2 Fe
��2���� ����
��3��8Al+3Fe3O4 4Al2O3+9Fe
4Al2O3+9Fe
��4��3Fe2++4H++NO3 ��=3Fe3++NO��+2H2O
��1��H2O2 Fe
��2���� ����
��3��8Al+3Fe3O4
 4Al2O3+9Fe
4Al2O3+9Fe��4��3Fe2++4H++NO3 ��=3Fe3++NO��+2H2O
��

��ϰ��ϵ�д�
�����Ŀ

 ��
��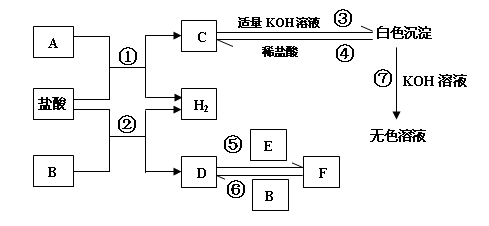

 g
g