��Ŀ����
����Ŀ��[��ѧ����ѡ��5���л���ѧ����]
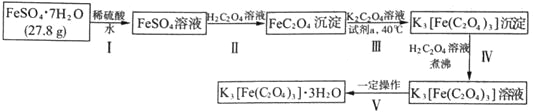
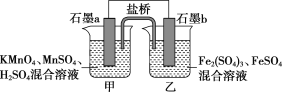
�ײ������ᣬ�����Ǽ������ᣬ�ھ�ϸ�����������Ź㷺����;����ϳ�·�����£�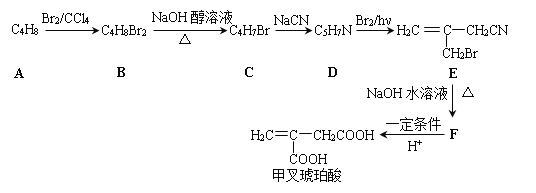
��֪��![]()
�ش��������⣺
��1���ײ��������й����ŵ�����Ϊ_______��_______��
��2��A������Ϊ_______��A��B�Ļ�ѧ����ʽΪ_______��
��3��B��C�Ļ�ѧ����ʽΪ_______��C��D�ķ�Ӧ����Ϊ_______��
��4��F�Ľṹ��ʽΪ_______��
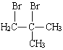
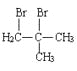
��5���ײ��������ͬ���칹��MҲ�Dz����Ͷ�Ԫ���ᣬ��M���� _______�֣������������칹�������к˴Ź���������ʾ����壬�ҷ����֮��Ϊ3��1��2�Ľṹ��ʽΪ _______��
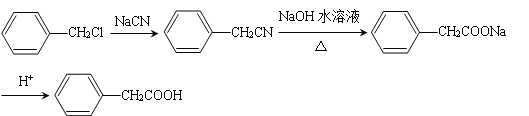
��6������������Ϣ��д����һ�ȼױ���![]() CH2Cl��Ϊԭ�ϣ����Լ���ѡ���Ʊ������ᣨ
CH2Cl��Ϊԭ�ϣ����Լ���ѡ���Ʊ������ᣨ![]() CH2COOH���ĺϳ�·��_______��
CH2COOH���ĺϳ�·��_______��
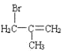
���𰸡��Ȼ� ̼̼˫�� 2-����ϩ ![]() +Br2
+Br2![]()
 +NaOH
+NaOH![]()
 +NaBr+H2O ȡ����Ӧ
+NaBr+H2O ȡ����Ӧ 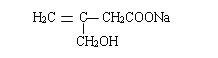 4 CH3CH=C(COOH)2
4 CH3CH=C(COOH)2 ![]()
![]()
![]()
![]()
![]()
![]()
![]()
��������
A�ķ���ʽΪC4H8�����巴Ӧ�õ�B��B������ȥ��Ӧ�õ�C��C����NaCN��Ӧ�������Ϣ�ڿ�֪C�л�����Brԭ�ӣ���AΪϩ������B��ֻ��1��Brԭ�ӿ��Է�����ȥ��Ӧ������֪AΪ![]() ��BΪ
��BΪ ��CΪ
��CΪ ��DΪ
��DΪ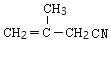 �������Ϣ�ܼ��ײ�������Ľṹ����֪D�м���Hԭ����ȫ������ԭ��ȡ������E����EΪ
�������Ϣ�ܼ��ײ�������Ľṹ����֪D�м���Hԭ����ȫ������ԭ��ȡ������E����EΪ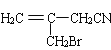 ��FΪ
��FΪ![]() ��F�ữˮ��õ��ײ������ᡣ
��F�ữˮ��õ��ײ������ᡣ
��1�����ݼײ�������ṹ��֪�����й����ŵ�����Ϊ�Ȼ���̼̼˫�����ʴ�Ϊ���Ȼ���̼̼˫����
��2��A�ܺ��巢���ӳɷ�Ӧ��A�к���˫��������E����˵��������3��̼ԭ�ӣ���A������Ϊ2-����ϩ�������ˮ�����ӳɷ�Ӧ�ķ���ʽΪ��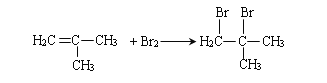 ���ʴ�Ϊ��2-����ϩ��
���ʴ�Ϊ��2-����ϩ��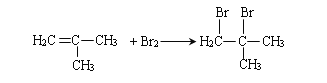 ��
��
��3���������֪B��C�Ƿ�����ȥ��Ӧ����˫�����仯ѧ����ʽΪ  +NaOH
+NaOH![]()
 +NaBr+H2O��C��D�ķ�Ӧ���������ȡ����ԭ������ȡ����Ӧ���ʴ�Ϊ��
+NaBr+H2O��C��D�ķ�Ӧ���������ȡ����ԭ������ȡ����Ӧ���ʴ�Ϊ�� +NaOH
+NaOH![]()
 +NaBr+H2O��ȡ����Ӧ��
+NaBr+H2O��ȡ����Ӧ��
��4������������Ϣ��֪��F���������������ɼײ������ᣬF��Ϊ�����ƣ�F�Ľṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5���ײ��������ͬ���칹��MҲ�Dz����Ͷ�Ԫ���ᣬ��M����4�֣����к˴Ź���������ʾ����壬�ҷ����֮��Ϊ3��1��2�Ľṹ��ʽΪ��CH3CH=C(COOH)2���ʴ�Ϊ��4��CH3CH=C(COOH)2��
��6�������軯�Ʒ�Ӧ����  ��Ȼ������������ˮ��Һ��������
��Ȼ������������ˮ��Һ��������  ���������������������
��������������������� ![]() ������
������ ��
��

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�����Ŀ���ڻ�ƿ�м������ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
�ɷ� | ������g�� | Ħ��������g ��mol-1�� |
���� | 25��00 | 342 |
����� | 0��25 | 174 |
��˾ƥ�� | 0��17 | 180 |
������� | 0��25 | 158 |
������ | 0��02 | 170 |
��1���������ʻ����ʼ����ijɷ��У����ڷǵ���ʵ���________��
A������ B������� C��������� D��������
��2�����ʻ����ʼ�����K+����˾ƥ���в���K+�������ʵ���Ũ��Ϊ_______ mol/L����ע�⣺ֻҪ����ԭʼ����д����ʽ������Ҫ��������㣩
��3�������������ʻ����ʼ�������������У��ձ���������ƽ��ҩ�ס�________________��______________��_______________�����ں�������д��ȱ���������ƣ�
��4������Һ���ƹ����У����в��������ƽ��û��Ӱ�����___________��
A������ʱ��������ƿ�̶���
B������ƿ��ʹ��ǰδ�����������������ˮ
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�NaCl��Һ��δϴ��
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ���