��Ŀ����
16���Ƚ����л�ѧ��Ӧ��������Һ�и�����Ũ�ȵĴ�С����10mL��0.2mol•L-1��HCl��10mL��0.2mol•L-1��ˮ���������Һ�и�����Ũ���ɴ�С��˳����c��Cl-����c��NH4+����c��H+����c��OH-����
��10mL��0.4mol•L-1��CH3COONa��Һ��10mL��0.2mol•L-1��HCl��Һ��Ϻ�������Һ�и�����Ũ���ɴ�С��˳����c��Na+����c��CH3COO-����c��Cl-����c��H+����c��OH-����
���� ��10mL��0.2mol•L-1��HCl��10mL��0.2mol•L-1��ˮ���������ҺΪ�Ȼ����Һ��笠�����ˮ����Һ�����ԣ��ݴ˷����ж���Һ������Ũ�ȴ�С��
��10mL��0.4mol•L-1��CH3COONa��Һ��10mL��0.2mol•L-1��HCl��Һ��Ϻõ�Ϊ��Ũ�ȵĴ��ᡢ�Ȼ��ƺʹ����ƻ����Һ����Һ�д��������ڴ�������ӵ�ˮ�⣬��Һ�����ԣ�
��� �⣺��10mL��0.2mol•L-1��HCl��10mL��0.2mol•L-1��ˮ���������ҺΪ�Ȼ����Һ��笠�����ˮ����Һ�����ԣ��ݴ˷����ж���Һ������Ũ�ȴ�СΪ��c��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
��10mL��0.4mol•L-1��CH3COONa��Һ��10mL��0.2mol•L-1��HCl��Һ��Ϻõ�Ϊ��Ũ�ȵĴ��ᡢ�Ȼ��ƺʹ����ƻ����Һ����Һ�д��������ڴ�������ӵ�ˮ�⣬��Һ�����ԣ���Һ������Ũ�ȴ�СΪ��c��Na+����c��CH3COO-����c��Cl-����c��H+����c��OH-����
���� ���⿼���˵������Һ������Ũ�ȴ�С�Ƚϣ���Ҫ����Һ�����ʳɷֵķ����жϣ���������ˮ���������ʵ���ij̶ȷ����ж��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

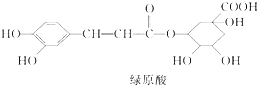
���й�����ԭ���жϲ���ȷ���ǣ�������
| A�� | ��ԭ����Ժ�NaHCO3��Ӧ���ų�CO2 | |
| B�� | 1mol��ԭ����������ˮ��Ӧ���������2.5molBr2 | |
| C�� | 1mol��ԭ��������NaOH��Һ��Ӧ���������4molNaOH | |
| D�� | ��ԭ��ˮ������������FeCl3��Һ������ɫ��Ӧ |
| A�� | 3Mg•Ca•4Si•6O2 | B�� | MgO•CaO•SiO2 | C�� | 3MgO•CaO•4SiO2 | D�� | Mg3Ca��SiO3��4 |

��֪��
| ���� | ��Է������� | ��ɫ��״̬ | �е㣨�棩 | �ܶȣ�g•cm-3�� |
| ������* | 122 | ��ɫƬ״���� | 249 | 1.2659 |
| ���������� | 150 | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | 46 | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | 84 | ��ɫ����Һ�� | 80.8 | 0.7318 |
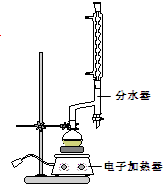 ʵ�鲽�����£�
ʵ�鲽�����£�����Բ����ƿ�м���12.20g�����ᣬ25mL 95%���Ҵ�����������20mL�������Լ�4mLŨ���ᣬ��Ͼ��Ȳ������ʯ����ͼ��ʾװ�������������¶���65��70����Ȼ���2h�����÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
�ڷ�Ӧ�������������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȣ�
�۽���ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������Na2CO3����Һ�������ԣ��÷�Һ©���ֳ��л��㣬ˮ����25mL������ȡ��Һ��Ȼ��ϲ����л��㣬�����Ȼ��ƣ����ã����ˣ�����Һ�����������������Ѻͻ�����������£�����210��213�����֣�
�ܼ���ϸ�ò�Ʒ���Ϊ12.86mL��
�ش��������⣺
��1���ڸ�ʵ���У�Բ����ƿ���ݻ����ʺϵ��ǣ�������ȷѡ��ǰ����ĸ��C��
A��25mL B��50mL C��100mL D��250mL
��2���������ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����ʹƽ�ⲻ�ϵ��������ƶ���
��3���������Ӧ������ֵ��¶���C��
A��65��70��B��78��80��C��85��90��D��215��220��
��4������ۼ���Na2CO3�������dz�ȥ�����������еı������Na2CO3���벻�㣬��֮������ʱ��������ƿ�пɼ����������ɣ������������ԭ�����ڱ�������������δ�����ı����ᣬ������100��ʱ������
��5�����ڲ�����е���ȡ��Һ����������ȷ����AD
A��ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ���������Һ©����ת������������ҡ
B����ҡ���κ����Һ©���ϿڵIJ���������
C����������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D���ų�Һ��ʱ��Ӧ���Ͽڲ������������ϵİ��۶�©�����ϵ�С��
��6�����㱾ʵ��IJ���Ϊ90.02%��
| A�� | 2.8mol | B�� | 1.6mol | C�� | 3.2mol | D�� | 3.6mol |
| A�� | 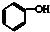 ʯ̿�� ʯ̿�� | B�� |  TNT TNT | ||
| C�� | CH3COOH ���� | D�� | CH2BrCH2Br 1��2-�������� |
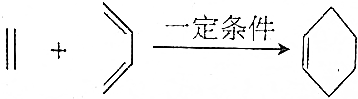
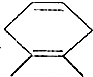 ����ش��������⣺
����ش��������⣺