��Ŀ����
����ij�л���ķ���ʽC9H18O2��������������ˮ��Ϊ�Һͱ������л������ͬ�¶Ⱥ�ѹǿ�£�ͬ�������Һͱ���������ռ�����ͬ����Ŀ��ܽṹ��_________�֡�����ij�л���Qֻ��̼���⡢������Ԫ�أ����ⶨ������̼���⡢��ԭ�Ӹ�����Ϊ10��16��3������Է�������������200����֪Q����NaHCO3��Һ��Ӧ�����л����ת����ϵ����ͼ��ʾ��
��֪��
R��CH=CH��R��![]() R��COOH+R�䡪COOH
R��COOH+R�䡪COOH
R��CH=CH��R��![]() R��CHO+R�䡪CHO
R��CHO+R�䡪CHO

��1��д��Q�Ľṹ��ʽ__________________________________________________________
��2��д����Ӧ�۵Ļ�ѧ����ʽ____________________________________________________
��3��д����Ӧ�ܵ����ӷ���ʽ____________________________________________________
��4��д����D������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ__________________������һ�֣�
��:16��

��3��CH3CHO+2Ag(NH3)2++2OH-![]() CH3COO-+
CH3COO-+![]() +2Ag��+3NH3��+H2O
+2Ag��+3NH3��+H2O
��4��![]()
����������ȷ�𰸣�
����������C9H18O2+H2O![]() CaH
CaH![]() ��
��![]() ��
��![]() ��
�� ��
��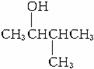 ��
��![]() ��
��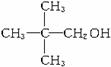 ����һˮ��������ʽΪC4H8O2����2��ͬ���칹�壺CH3CH2CH2COOH��
����һˮ��������ʽΪC4H8O2����2��ͬ���칹�壺CH3CH2CH2COOH��![]() ����ˮ������Ӧ��������8��2=16��ͬ���칹�塣
����ˮ������Ӧ��������8��2=16��ͬ���칹�塣
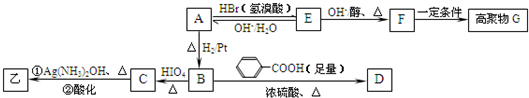
���������Ag(NH3)2��+��Ӧ��C2H4OΪCH3CHO����֪C��Ƭ�ϡ�CH3CH=�������DΪֱ���������֪CΪֱ���������Q��A��B��E��F��Ϊֱ���������Q�ķ���ʽΪC10aH16aO![]() ����֪Q��Ƭ��HOOC��CH=����֪E��HOOC����F�ຬHOOC������F���ɵ���GΪ��Ԫ��״�������֪FΪ
����֪Q��Ƭ��HOOC��CH=����֪E��HOOC����F�ຬHOOC������F���ɵ���GΪ��Ԫ��״�������֪FΪ![]() ����F��E��NaBH4��ԭ���ɣ�֪E��ͪ������EΪ
����F��E��NaBH4��ԭ���ɣ�֪E��ͪ������EΪ![]() ����һ����֪QΪ
����һ����֪QΪ![]() ��
��
AΪ![]() ��
��
BΪ![]() ��
��
CΪCH3CH=CH��CH2��6COOH��
DΪOHC����CH2��6��COOH��
HΪCH3COONH4��
FΪ![]() ��
��
GΪ 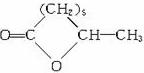 ��
��

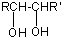



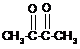
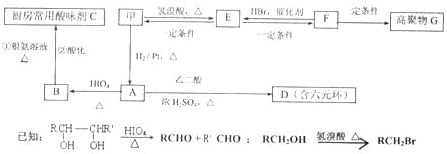


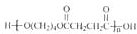
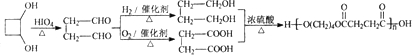
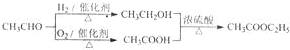






 ��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��
��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��