��Ŀ����
��2011?������һģ�������£�������������Һ��
|
������A.0.1mol/LNaOH��Һ�У���ˮ�������c��H+��Ϊ10-13mol/L��0.1mol/L CH3COOH��Һ��c��H+����0.1mol/L������ˮ�������c��H+������10-13mol/L��
B.0.1mol/L CH3COOH��Һ��c��H+����0.1mol/L����pH��1������Ϊ3��ϡ��100����pH����
C������ۻ�ϣ�����ҺpH=7��c��H+��=c��OH-������ϵ���غ������
D������ܻ�ϣ�����Һ�����ԣ���Ϊ�����������Ļ����Һ�����������ڴ��������ˮ�⣬������϶࣬��c��H+����c��Na+����
B.0.1mol/L CH3COOH��Һ��c��H+����0.1mol/L����pH��1������Ϊ3��ϡ��100����pH����
C������ۻ�ϣ�����ҺpH=7��c��H+��=c��OH-������ϵ���غ������
D������ܻ�ϣ�����Һ�����ԣ���Ϊ�����������Ļ����Һ�����������ڴ��������ˮ�⣬������϶࣬��c��H+����c��Na+����
����⣺A.0.1mol/LNaOH��Һ�У���ˮ�������c��H+��Ϊ10-13mol/L��0.1mol/L CH3COOH��Һ��c��H+����0.1mol/L������ˮ�������c��H+������10-13mol/L��������ˮ�������c��H+��Ϊ�٣��ۣ���A����
B.0.1mol/L CH3COOH��Һ��c��H+����0.1mol/L����pH��1������Ϊ3��ϡ��100����pH������۵�pH������ܲ�ͬ����B����
C������ۻ�ϣ�����ҺpH=7��c��H+��=c��OH-�����ɵ���غ��֪c��CH3COO-��=c��Na+������Ϊ����ʹ����ƵĻ��Һ������Ũ�ȴ���NaOH��Һ��Ũ�ȣ��������������ɣ�����V��NaOH����V��CH3COOH������V��NaOH����V��CH3COOH��Ҳ���ܣ���C����
D������ܻ�ϣ�����Һ�����ԣ���Ϊ�����������Ļ����Һ�����������ڴ��������ˮ�⣬������϶࣬��c��H+����c��Na+������ϵ���غ��֪��Һ������Ũ�ȿ���Ϊc��CH3COO-����c��H+����c��Na+����c��OH-������D��ȷ��
��ѡD��
B.0.1mol/L CH3COOH��Һ��c��H+����0.1mol/L����pH��1������Ϊ3��ϡ��100����pH������۵�pH������ܲ�ͬ����B����
C������ۻ�ϣ�����ҺpH=7��c��H+��=c��OH-�����ɵ���غ��֪c��CH3COO-��=c��Na+������Ϊ����ʹ����ƵĻ��Һ������Ũ�ȴ���NaOH��Һ��Ũ�ȣ��������������ɣ�����V��NaOH����V��CH3COOH������V��NaOH����V��CH3COOH��Ҳ���ܣ���C����
D������ܻ�ϣ�����Һ�����ԣ���Ϊ�����������Ļ����Һ�����������ڴ��������ˮ�⣬������϶࣬��c��H+����c��Na+������ϵ���غ��֪��Һ������Ũ�ȿ���Ϊc��CH3COO-����c��H+����c��Na+����c��OH-������D��ȷ��
��ѡD��
���������⿼�������pH���жϼ���Һ����Եķ�����ѡ��CΪ�����ѵ㣬ע�������ʱpH��Ũ�ȵĹ�ϵ��������ˮ��Ĺ�ϵ�ȼ��ɽ����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
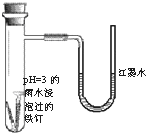 ��2011?������һģ����ͼ��̽����������ʴ��װ��ͼ�����ֿ�ʼʱU����˺�īˮˮ���½���һ��ʱ���U����˺�īˮˮ��������������˵������ȷ���ǣ�������
��2011?������һģ����ͼ��̽����������ʴ��װ��ͼ�����ֿ�ʼʱU����˺�īˮˮ���½���һ��ʱ���U����˺�īˮˮ��������������˵������ȷ���ǣ������� ��2011?������һģ��X��Y��Z��W��Ϊ������Ԫ�أ������ڱ���λ����ͼ��ʾ��Yԭ�ӵ������������ǵ��Ӳ�����3��������˵���в���ȷ���ǣ�������
��2011?������һģ��X��Y��Z��W��Ϊ������Ԫ�أ������ڱ���λ����ͼ��ʾ��Yԭ�ӵ������������ǵ��Ӳ�����3��������˵���в���ȷ���ǣ�������