��Ŀ����
����Ŀ���߷��ӻ�����M�ĺϳ�·�����£�

��֪��![]()
(1)A�к��������ŵ�������______��
(2)H��A��ͬ���칹�壬����FeCl3��ʾ�������ɫ���˴Ź���������ʾH��5��壬����ṹ��ʽΪ____________________��
(3) B�Ľṹ��ʽΪ______��G�Ľṹ��ʽΪ__________________��
(4) ��֪DΪ����������2 D �� E + C2H5OH��F�к��д��ǻ����ݵķ�Ӧ����Ϊ_____��
(5) д���ܵķ�Ӧ����ʽ___________________________________________��
(6) ����ȩΪԭ�ϣ�д���ϳ�D������ͼ______��(�������Լ���ѡ)��
���𰸡��ʻ� ![]()
 ��ȥ��Ӧ
��ȥ��Ӧ  +
+ 
![]()
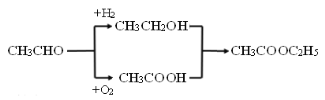
��������
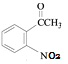
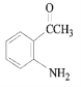
������Ϣ����Ӧ�ڷ�����ԭ��Ӧ����NO2����NH2����B�Ľṹ��ʽΪ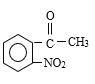 ����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ
����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ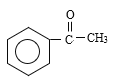 ������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ
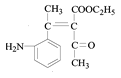
������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ ���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ
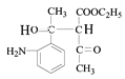
���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ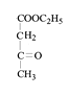 ��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ
��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ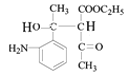 ����Ӧ��������ȥ��Ӧ��
����Ӧ��������ȥ��Ӧ��
������Ϣ����Ӧ�ڷ�����ԭ��Ӧ����NO2����NH2����B�Ľṹ��ʽΪ ����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ
����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ ������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ
������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ ���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ
���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ ��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ
��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ ����Ӧ��������ȥ��Ӧ��
����Ӧ��������ȥ��Ӧ��
��1����������������A�к������������ʻ���
��2������FeCl3������ɫ��Ӧ��˵�����ǻ���H��Ӧ����̼̼˫�����˴Ź���������5��壬˵����5�ֲ�ͬ�������⣬Ӧ�ǶԳƽṹ�����ṹ��ʽΪ![]() ��
��
��3����������������B�Ľṹ��ʽΪ ��G�Ľṹ��ʽΪ
��G�Ľṹ��ʽΪ ��
��
��4��F��G������span>OH������̼̼˫��������Ӧ�ݵ�����Ϊ��ȥ��Ӧ��
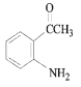
��5����Ӧ��Ϊ�ӳɷ�Ӧ���䷴Ӧ����ʽΪ +
+ 
![]()
 ��
��
��6��DΪ�����������ṹ��ʽΪCH3COOCH2CH3������ȩ���������ᣬ����ȩ��H2�����ӳɷ�Ӧ�����Ҵ���Ȼ��������Ҵ�����������Ӧ���õ���������������ͼΪ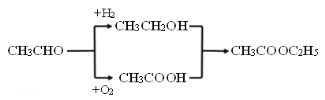 ��
��

����Ŀ���״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������ҵ��һ������������ַ�Ӧ�ϳɼ״�����Ӧ��CO(g)��2H2(g) ![]() CH3OH(g)����H1
CH3OH(g)����H1
��Ӧ��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g)����H2
CH3OH(g)��H2O(g)����H2
�±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)��
�¶� | 250 �� | 300 �� | 350 �� |
K | 2.0 | 0.27 | 0.012 |
(1)�ɱ��������жϦ�H1________(�����������������)0����ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g)����H3��___________(�æ�H1�ͦ�H2��ʾ)��
CO(g)��H2O(g)����H3��___________(�æ�H1�ͦ�H2��ʾ)��
(2)�������ݻ����䣬�����д�ʩ����߷�Ӧ����COת���ʵ���________(����ĸ���)
A������CO��ʹ��ϵ��ѹǿ���� B����CH3OH(g)����ϵ�з���
C������He��ʹ��ϵ��ѹǿ���� D��ʹ�ø�Ч����
(3)���ֺ��º����������ڷ�Ӧ��:��10 mol CO2��30mol H2����1 L���ܱ������У���ַ�Ӧ����CO2��ת����Ϊ60%����÷�Ӧ��ƽ�ⳣ��Ϊ____________________����ά�������������������Ͷ��10 mol CO2��30mol H2��10 mol CH3OH(g)�� 10mol H2O(g)���ж�ƽ���ƶ��ķ�����________(������ƶ������������ƶ������ƶ���)��
(4)��Ӧ����淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ����ͼ��֪����Ӧ��t1��t3��t7ʱ���ﵽ��ƽ�⣬��t2��t8ʱ���ı���һ�����������жϸı��������t2ʱ_______________________��t8ʱ______________________________��




