��Ŀ����
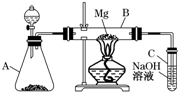 ijͬѧ����ͼ��ʾװ��̽��SO2�����ʼ����й�ʵ�飮
ijͬѧ����ͼ��ʾװ��̽��SO2�����ʼ����й�ʵ�飮��1��ʵ�������������ƹ����һ��Ũ�ȵ����ᷴӦ�Ʊ�SO2���壬д���÷�Ӧ�����ӷ���ʽ
SO32-+2H+�TSO2��+H2O
SO32-+2H+�TSO2��+H2O
��2���ֱ�SO2����ͨ������C��Һ�У���ش��������⣺������SO2ͨ����ɫʯ����Һ��������
���
���
������ͨ�����SO2���壬����������ɫ������
����ɫ������
����SO2ͨ����ɫKMnO4��Һ��������
��ɫ�䵭����ɫ��ʧ
��ɫ�䵭����ɫ��ʧ
���䷴Ӧ�����ӷ���ʽΪ5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+
5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+
���۹���SO2������ͨ�����ʯ��ˮ�У�����
�ȱ���ǣ����ֱ����
�ȱ���ǣ����ֱ����
���ӷ���ʽ��Ca2++2OH-+SO2=CaSO3 ��+H2O��CaSO3+SO2+H20=Ca2++2HSO3-��
Ca2++2OH-+SO2=CaSO3 ��+H2O��CaSO3+SO2+H20=Ca2++2HSO3-��
����CΪ˫��ˮ����ͨ��SO2��������Һ��
H2SO4
H2SO4
�������ʵĻ�ѧʽ��������ͬѧ�Ʊ���SO2�����л���CO2���壬�������ʵ�ԭ��������������ƹ����л���̼���λ�̼������
̼���λ�̼������
����������1���������ƺ����ᷴӦ���ɶ������������ƺ�ˮ��ǿ����ȡ���ᣮ
��2���ٶ��������ˮ��Ӧ���������ᣬ��ɫʯ����Һ������ɫ������������Ư���ԣ�������ʹʯ����ɫ��
�ڶ��������л�ԭ�ԣ����������ǿ�����ԣ����Զ��������������ܷ���������ԭ��Ӧ��ʹ���������ɫ��
�۶�������Ͷ�����̼�������ԣ�����ʹ�����ʯ��ˮ����ǣ�����ͨ����������������������Ʒ�Ӧ���ɿ����Ե���������ƣ�
�ܶ��������л�ԭ�ԣ�˫��ˮ��ǿ�����ԣ����Զ��������˫��ˮ�ܷ���������ԭ��Ӧ�������ᣬ̼���κ�̼�������������ᷴӦ���ɶ�����̼���ݴ˷�����
��2���ٶ��������ˮ��Ӧ���������ᣬ��ɫʯ����Һ������ɫ������������Ư���ԣ�������ʹʯ����ɫ��
�ڶ��������л�ԭ�ԣ����������ǿ�����ԣ����Զ��������������ܷ���������ԭ��Ӧ��ʹ���������ɫ��
�۶�������Ͷ�����̼�������ԣ�����ʹ�����ʯ��ˮ����ǣ�����ͨ����������������������Ʒ�Ӧ���ɿ����Ե���������ƣ�
�ܶ��������л�ԭ�ԣ�˫��ˮ��ǿ�����ԣ����Զ��������˫��ˮ�ܷ���������ԭ��Ӧ�������ᣬ̼���κ�̼�������������ᷴӦ���ɶ�����̼���ݴ˷�����
����𰸣���1���������ƺ����ᷴӦ���ɶ������������ƺ�ˮ�����ӷ���ʽΪ��SO32-+2H+�TSO2��+H2O��
�ʴ�Ϊ��SO32-+2H+�TSO2��+H2O��
��2���ٶ��������ˮ��Ӧ���������ᣬ�������ܵ���������ƶ��������ӣ�������Һ�����ԣ���ɫʯ����Һ������ɫ��������Һ���죻����������Ư���ԣ�������ʹʯ����ɫ�����Լ���ͨ�����SO2���壬�����Dz���ɫ�����ԣ�
�ʴ�Ϊ����죻����ɫ�����ԣ�
�ڶ��������л�ԭ�ԣ����������ǿ�����ԣ����Զ��������������ܷ���������ԭ��Ӧ��ʹ���������Һ��ɫ��dz����ɫ��ʧ�����ӷ�Ӧ����ʽΪ��5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+��
�ʴ�Ϊ����ɫ�䵭����ɫ��ʧ��5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+��
�۶���������������Ʒ�Ӧ���ɲ�����ˮ��������ƺ�ˮ��������ƺͶ�������ˮ�ܷ�Ӧ���ɿ����Ե���������ƣ����ӷ�Ӧ����ʽΪ��Ca2++2OH-+SO2=CaSO3 ��+H2O��CaSO3+SO2+H20=Ca2++2HSO3-��
�ʴ�Ϊ���ȱ���ǣ����ֱ���壻Ca2++2OH-+SO2=CaSO3 ��+H2O��CaSO3+SO2+H20=Ca2++2HSO3-��
�ܶ��������л�ԭ�ԣ�˫��ˮ��ǿ�����ԣ����Զ��������˫��ˮ�ܷ���������ԭ��Ӧ�������̼���κ�̼�������������ᷴӦ���ɶ�����̼������ͬѧ�Ʊ���SO2�����л���CO2���壬�������ʵ�ԭ��������������ƹ����л���̼���λ�̼�����Σ�
�ʴ�Ϊ��H2SO4��̼���λ�̼�����Σ�
�ʴ�Ϊ��SO32-+2H+�TSO2��+H2O��
��2���ٶ��������ˮ��Ӧ���������ᣬ�������ܵ���������ƶ��������ӣ�������Һ�����ԣ���ɫʯ����Һ������ɫ��������Һ���죻����������Ư���ԣ�������ʹʯ����ɫ�����Լ���ͨ�����SO2���壬�����Dz���ɫ�����ԣ�
�ʴ�Ϊ����죻����ɫ�����ԣ�
�ڶ��������л�ԭ�ԣ����������ǿ�����ԣ����Զ��������������ܷ���������ԭ��Ӧ��ʹ���������Һ��ɫ��dz����ɫ��ʧ�����ӷ�Ӧ����ʽΪ��5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+��
�ʴ�Ϊ����ɫ�䵭����ɫ��ʧ��5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+��
�۶���������������Ʒ�Ӧ���ɲ�����ˮ��������ƺ�ˮ��������ƺͶ�������ˮ�ܷ�Ӧ���ɿ����Ե���������ƣ����ӷ�Ӧ����ʽΪ��Ca2++2OH-+SO2=CaSO3 ��+H2O��CaSO3+SO2+H20=Ca2++2HSO3-��
�ʴ�Ϊ���ȱ���ǣ����ֱ���壻Ca2++2OH-+SO2=CaSO3 ��+H2O��CaSO3+SO2+H20=Ca2++2HSO3-��
�ܶ��������л�ԭ�ԣ�˫��ˮ��ǿ�����ԣ����Զ��������˫��ˮ�ܷ���������ԭ��Ӧ�������̼���κ�̼�������������ᷴӦ���ɶ�����̼������ͬѧ�Ʊ���SO2�����л���CO2���壬�������ʵ�ԭ��������������ƹ����л���̼���λ�̼�����Σ�
�ʴ�Ϊ��H2SO4��̼���λ�̼�����Σ�
���������⿼���˶�����������ʣ��ѶȲ���ע�����������Ư���ԣ�������������Ư��ʯ����Һ��

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д�
�����Ŀ

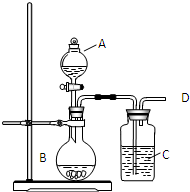 ijͬѧ����ͼ��ʾװ��̽��SO2�����ʼ����й�ʵ�飮
ijͬѧ����ͼ��ʾװ��̽��SO2�����ʼ����й�ʵ�飮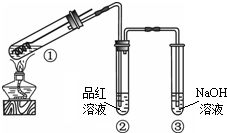 ijͬѧ����ͼ��ʾװ�ã��г���������ȥ��̽��ͭ˿�����Ũ����ķ�Ӧ��
ijͬѧ����ͼ��ʾװ�ã��г���������ȥ��̽��ͭ˿�����Ũ����ķ�Ӧ�� ijͬѧ����ͼ��ʾװ����ȡ�������������Թܼ��м����������Ҵ���Ũ����ͱ����ᣬ������3min��5min����ش�
ijͬѧ����ͼ��ʾװ����ȡ�������������Թܼ��м����������Ҵ���Ũ����ͱ����ᣬ������3min��5min����ش�