��Ŀ����
��14�֣����ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ�������л�������У���ͬ��ԭ�ӵĺ˴Ź������и����ķ�ֵ���źţ�Ҳ��ͬ�����ݷ�ֵ���źţ�����ȷ���л����������ԭ�ӵ��������Ŀ����������ѵĽṹ��ʽΪ��
CH3��CH2��O��CH2��CH3
��˴Ź������и����ķ�ֵ���źţ�����������ͼ��ʾ��
��1�����������У���˴Ź��������и����ķ�ֵ���źţ�ֻ��һ������ ����ѡ�۷֣���
A��CH3CH3 B��CH3COOH
C��CH3COOCH3 D��CH3OCH3
��2��������A��B�ķ���ʽ����C2H4Br2, A�ĺ˴Ź�������ͼ����ͼ��ʾ����A�Ľṹ��ʽΪ�� ����Ԥ��B�ĺ˴Ź����������� ���壨�źţ���
��3����֪ij��C��H��O����Ԫ�ص�δ֪��R����ȼ�շ���ʵ��ⶨ��δ֪��̼����������Ϊ52.16%�������������Ϊ13.14%����R����Է�������Ϊ46������R�ķ���ʽ__________���ú˴Ź������ķ������о�R�Ľṹ����Ҫ˵�����ݺ˴Ź������Ľ����ȷ��R���ӽṹ�ķ�����
��
��1��AD��4�֣���(ȫ�Ը�4��,ֻѡһ����2��,ѡ��һ������1��)
��2��BrCH2CH2Br��2�֣��� 2��2�֣���
��3��C2H6O(2��)
��ͼ���и�����3�����շ壨�źţ�����˵��C2H6O�Ľṹ��CH3CH2OH��2�֣���
��ͼ���и�����1�����շ壨�źţ�����˵��C2H6O�Ľṹ��CH3OCH3��2�֣�
����:

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� ����˴Ź�����������2���źţ��μ�ͼ����
����˴Ź�����������2���źţ��μ�ͼ����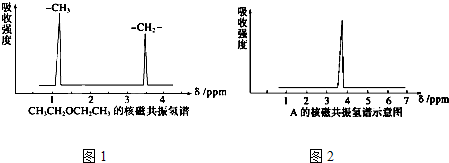
 ���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ����
���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ���� ���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ�������л�������У���ͬ��ԭ�ӵĺ˴Ź������и����ķ�ֵ���źţ�Ҳ��ͬ�����ݷ�ֵ���źţ�����ȷ���л����������ԭ�ӵ��������Ŀ��
���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ�������л�������У���ͬ��ԭ�ӵĺ˴Ź������и����ķ�ֵ���źţ�Ҳ��ͬ�����ݷ�ֵ���źţ�����ȷ���л����������ԭ�ӵ��������Ŀ��