��Ŀ����
 ���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ����
���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ������1�����������У���˴Ź��������и����ķ�ֵ���źţ�ֻ��һ������
AD
AD
A��CH3CH3 B��CH3COOH C��CH3COOCH3 D��CH3OCH3
��2��������A��B�ķ���ʽ����C2H4Br2��A�ĺ˴Ź�������ͼ��ͼ��ʾ����д��B�Ľṹ��ʽ
CH3CHBr2
CH3CHBr2
��3���ú˴Ź������ķ������о�C2H6O�Ľṹ����Ҫ˵�����ݺ˴Ź������Ľ����ȷ��C2H6O���ӽṹ�ķ���
��ͼ���и�����3�����շ壬��˵��C2H6O�Ľṹ��CH3CH2OH
��ͼ���и�����3�����շ壬��˵��C2H6O�Ľṹ��CH3CH2OH
��ͼ���и�����1�����շ壨�źţ�����˵��C2H6O�Ľṹ��CH3OCH3
��ͼ���и�����1�����շ壨�źţ�����˵��C2H6O�Ľṹ��CH3OCH3
����������1���˴Ź��������з�ֵ�������л�������ԭ�ӵ����������˴Ź���������ֻ����һ�ַ壬˵���÷����е�Hԭ�Ӷ��ǵ�Ч�ģ�ֻ��1��Hԭ�ӣ�
��2��������C2H4Br2�Ľṹ�����֣�CH3CHBr2���������͵���ԭ�ӣ�CH2Br-CH2Brֻ��һ�����͵���ԭ�ӣ�
��3��C2H6O�Ľṹ�����֣�CH3CH2OH��CH3OCH3��
��2��������C2H4Br2�Ľṹ�����֣�CH3CHBr2���������͵���ԭ�ӣ�CH2Br-CH2Brֻ��һ�����͵���ԭ�ӣ�
��3��C2H6O�Ľṹ�����֣�CH3CH2OH��CH3OCH3��
����⣺��1���˴Ź���������ֻ����һ�ַ壬˵���÷����е�Hԭ�Ӷ��ǵ�Ч�ģ�ֻ��1��Hԭ�ӣ�
A��CH3CH3��6��Hԭ�Ӷ��ǵ�Ч�ģ��˴Ź���������ֻ����һ�ַ壬��A��ȷ��
B��CH3COOH�м��е�Hԭ�����Ȼ��е�Hԭ��������ѧ������ͬ��CH3COOH��2��Hԭ�ӣ��˴Ź�����������2���壬��B����
C��CH3COOCH3�������Ļ�ѧ������ͬ���˴Ź�����������2���壬��C����
D��CH3OCH3��2��������ͬһ����ԭ���ϣ�6��Hԭ�Ӷ��ǵ�Ч�ģ��˴Ź���������ֻ����һ�ַ壬��D��ȷ��
��ѡAD��
��2��������C2H4Br2�Ľṹ�����֣�CH3CHBr2���������͵���ԭ�ӣ�CH2Br-CH2Brֻ��һ�����͵���ԭ�ӣ���A�ĺ˴Ź��������ж�A�Ľṹ��ʽΪCH2Br-CH2Br��
�ʴ�Ϊ��CH3CHBr2��
��3��C2H6O�Ľṹ�����֣�CH3CH2OH��CH3OCH3����ΪCH3CH2OH�����ں˴Ź�����������3���壮��ΪCH3OCH3�����ں˴Ź�����������1���壬
�ʴ�Ϊ����ͼ���и�����3�����շ壬��˵��C2H6O�Ľṹ��CH3CH2OH����ͼ���и�����1�����շ壨�źţ�����˵��C2H6O�Ľṹ��CH3OCH3��
A��CH3CH3��6��Hԭ�Ӷ��ǵ�Ч�ģ��˴Ź���������ֻ����һ�ַ壬��A��ȷ��
B��CH3COOH�м��е�Hԭ�����Ȼ��е�Hԭ��������ѧ������ͬ��CH3COOH��2��Hԭ�ӣ��˴Ź�����������2���壬��B����
C��CH3COOCH3�������Ļ�ѧ������ͬ���˴Ź�����������2���壬��C����
D��CH3OCH3��2��������ͬһ����ԭ���ϣ�6��Hԭ�Ӷ��ǵ�Ч�ģ��˴Ź���������ֻ����һ�ַ壬��D��ȷ��
��ѡAD��
��2��������C2H4Br2�Ľṹ�����֣�CH3CHBr2���������͵���ԭ�ӣ�CH2Br-CH2Brֻ��һ�����͵���ԭ�ӣ���A�ĺ˴Ź��������ж�A�Ľṹ��ʽΪCH2Br-CH2Br��
�ʴ�Ϊ��CH3CHBr2��
��3��C2H6O�Ľṹ�����֣�CH3CH2OH��CH3OCH3����ΪCH3CH2OH�����ں˴Ź�����������3���壮��ΪCH3OCH3�����ں˴Ź�����������1���壬
�ʴ�Ϊ����ͼ���и�����3�����շ壬��˵��C2H6O�Ľṹ��CH3CH2OH����ͼ���и�����1�����շ壨�źţ�����˵��C2H6O�Ľṹ��CH3OCH3��
���������⿼��˴Ź�������ȷ�����ӽṹ���ѶȲ���ע��˴Ź��������з�ֵ�������л�������ԭ�ӵ���������

��ϰ��ϵ�д�
�����Ŀ
 ����˴Ź�����������2���źţ��μ�ͼ����
����˴Ź�����������2���źţ��μ�ͼ����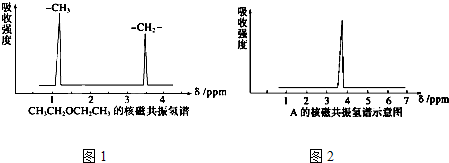
 ���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ�������л�������У���ͬ��ԭ�ӵĺ˴Ź������и����ķ�ֵ���źţ�Ҳ��ͬ�����ݷ�ֵ���źţ�����ȷ���л����������ԭ�ӵ��������Ŀ��
���ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ�������л�������У���ͬ��ԭ�ӵĺ˴Ź������и����ķ�ֵ���źţ�Ҳ��ͬ�����ݷ�ֵ���źţ�����ȷ���л����������ԭ�ӵ��������Ŀ��