��Ŀ����
����Ŀ����ͼ��ʵ��������ͭ��Ũ������ȡ��������̽���������ʣ���ش��������⣺
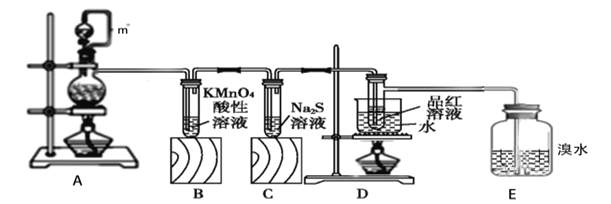
��1��װ��A��m������___��װ��A�������Ե�һ������___��
��2��װ��A�з����Ļ�ѧ��Ӧ����ʽ___���÷�Ӧ������������__________��
��3��װ��B�е�����________��֤��SO2����________��
��4��װ��C����Һ�ڿ����в��ױ��棬ʱ�䳤�˻���ֻ��ǣ�ԭ����_______���������ӷ���ʽ��ʾ��
��5��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ�д��ʵ�����������__��
��6��E�е�������___�������Ļ�ѧ��Ӧ����ʽ_______��
���𰸡�ƽ����ѹ��ʹҺ��˳���� ������ƿ�е�Һ�峬�����������������2/3 Cu+2H2SO4(Ũ)![]() CuSO4+2SO2��+2H2O CuSO4 KMnO4 ��Һ��ɫ����ɫ�����ɫ�� ��ԭ�� 2S2-+O2+2H2O��2S��+4OH- Ʒ����Һ��ɫ�رշ�Һ©�����������ٵ�ȼ�ƾ��ƣ���Һ��ɺ�ɫ ��ˮ��ɫ SO2+Br2+2H2O=2HBr+H2SO4
CuSO4+2SO2��+2H2O CuSO4 KMnO4 ��Һ��ɫ����ɫ�����ɫ�� ��ԭ�� 2S2-+O2+2H2O��2S��+4OH- Ʒ����Һ��ɫ�رշ�Һ©�����������ٵ�ȼ�ƾ��ƣ���Һ��ɺ�ɫ ��ˮ��ɫ SO2+Br2+2H2O=2HBr+H2SO4
��������
��1��װ��A��m������Ϊƽ����ѹ��ʹҺ��˳���£�����������ƿʹ�ù���������ƿ����ʢ��Һ�岻�ܳ������ݻ���2/3��Ҳ��������1/3��ͼ���Ѿ��������ݻ���2/3��
��Ϊ��ƽ����ѹ��ʹҺ��˳���£�������ƿ�е�Һ�峬�����������������2/3��
��2��װ��A��ͭ��Ũ������ȡ�����������Ļ�ѧ��Ӧ����ʽΪCu+2H2SO4(Ũ)![]() CuSO4+2SO2��+2H2O���÷�Ӧ��Cu��0�۱�Ϊ+2�ۣ�ʧ���ӣ�������������������CuSO4��
CuSO4+2SO2��+2H2O���÷�Ӧ��Cu��0�۱�Ϊ+2�ۣ�ʧ���ӣ�������������������CuSO4��
��Ϊ��Cu+2H2SO4(Ũ)![]() CuSO4+2SO2��+2H2O��CuSO4��
CuSO4+2SO2��+2H2O��CuSO4��
��3��װ��A������SO2���壬SO2���л�ԭ�ԣ����徭��װ��B�е�����KMnO4 ��Һ����������ԭ��Ӧ��KMnO4 ��Һ��ɫ����ɫ�����ɫ����
��Ϊ��KMnO4 ��Һ��ɫ����ɫ�����ɫ������ԭ�ԣ�
��4��װ��C��ΪNa2S��Һ��S2-���л�ԭ�ԣ������ױ�������O2�������ɵ���S��ʱ�䳤�˻���ֻ��ǣ������ӷ���ʽ��ʾ��2S2-+O2+2H2O��2S��+4OH-��
��Ϊ2S2-+O2+2H2O��2S��+4OH-
��5��װ��D��Ŀ����̽��SO2��Ʒ�����õ�Ư�����ǿ���ģ�ʵ�����������Ϊ��Ʒ����Һ��ɫ�رշ�Һ©�����������ٵ�ȼ�ƾ��Ƽ��ȣ���Һ��ɺ�ɫ��
��6�����ɵ�SO2ͨ�뵽E��SO2����ˮ����������ԭ��Ӧ����ˮ��Һ��ɫ�������Ļ�ѧ��Ӧ����ʽΪSO2+Br2+2H2O=2HBr+H2SO4��
��Ϊ����ˮ��Һ��ɫ��SO2+Br2+2H2O=2HBr+H2SO4

����Ŀ����1����2L�ܱ������У���ʼͶ��4 molN2��6molH2��һ������������NH3��ƽ��ʱ���ı��¶Ȳ�õ����������ʾ����֪��T1<T2��
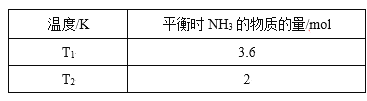
����K1______K2�����>������<����=����ԭ��_______��
����T2�£�����10s�ﵽ��ѧƽ��״̬����0��10s��N2��ƽ������v(N2)Ϊ______��ƽ��ʱH2��ת����Ϊ______������ͬʱ���Ӹ����ʵ���Ϊ1 mol���÷�Ӧ��ƽ��V��_____V����(>��=��<)ƽ�ⳣ����_____�����������С�����䡱��
��������˵���÷�Ӧ�Ѵﵽƽ��״̬����___��
A.3v��H2������2v��NH3���� B.����������ѹǿ����
C.��������ƽ����Է����������ٸı��״̬ D.��H���ֲ���
��2����֪���л�ѧ���ļ���д����ҵ���ư����Ȼ�ѧ����ʽ��
��ѧ�� | H-H | N��N | N-H |
����/kJ��mol-1 | 430 | 936 | 390 |
�Ȼ�ѧ����ʽ��__________��
��3�������ǿ���С����Ƶ�һ����������ԭ��أ����ߵ���Ӱ����Ϊa��b���Ե缫���ֱ��õ������ձ���m��n�����Ե缫�������ӣ�����ԭ��ʾ��ͼ��ͼ��
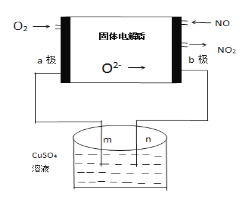
��aΪ___����b���ĵ缫��Ӧʽ____________��
���ڱ�״���£�ͨ��112mL��O2���ɹ۲쵽�ձ���n����_____���ɣ��������ձ��е���Һ�����Ϊ200mL��������䣩���Ӧ��ֹʱ�ձ�����Һ��PHΪ______��