��Ŀ����
(10��) ������һ����Ҫ�Ļ���ԭ�ϣ���ҵ����N2��H2�ϳ�NH3������֪N2(g)��H2(g)��Ӧ����1 mol NH3(g)�����������仯ʾ��ͼ������ͼ���ش��������⣺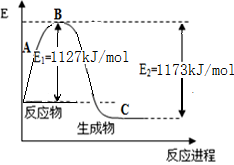
��1���÷�ӦΪ (����ȡ����ȡ�)��Ӧ��
��2���ϳɰ����Ȼ�ѧ����ʽΪ ��
��3��������֪�����������ϱ�����������������N-H������Ϊ kJ/mol��
��4����ҵ�ϣ�����Ϊԭ����������ĵ�һ��Ϊ������������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��1������ ��2��N2(g)+3H2(g) 2 NH3(g) ��H����92 kJ/mol
2 NH3(g) ��H����92 kJ/mol
��3��391 ��4�� 4NH3 + 5O2  4NO + 6H2O ��
4NO + 6H2O ��
���������������1�����ڷ�Ӧ����������������������������ʵ�����������ͷų�������˸÷�Ӧ�Ƿ��ȷ�Ӧ����2������ͼʾ��֪���ϳɰ����Ȼ�ѧ����ʽΪN2(g)+3H2(g) 2 NH3(g) ��H����92 kJ/mol����3����Ӧ�Ⱦ��Ƕ��ѻ�ѧ�����յ��������γɻ�ѧ���ͷŵ������IJ436 kJ/mol��3mol+946 kJ/mol��1mol-6X=��92 kJ/mol,���X=391 kJ/mol����4����ҵ�ϣ�����Ϊԭ����������ĵ�һ��Ϊ�����������÷�Ӧ�Ļ�ѧ����ʽ��4NH3 + 5O2
2 NH3(g) ��H����92 kJ/mol����3����Ӧ�Ⱦ��Ƕ��ѻ�ѧ�����յ��������γɻ�ѧ���ͷŵ������IJ436 kJ/mol��3mol+946 kJ/mol��1mol-6X=��92 kJ/mol,���X=391 kJ/mol����4����ҵ�ϣ�����Ϊԭ����������ĵ�һ��Ϊ�����������÷�Ӧ�Ļ�ѧ����ʽ��4NH3 + 5O2  4NO + 6H2O��
4NO + 6H2O��
���㣺���鷴Ӧ����ЧӦ����Ӧ������ܡ����ʵ������Ĺ�ϵ����ѧ����ʽ���Ȼ�ѧ����ʽ����д��֪ʶ��

��12�֣���1���������ı仯�ͷ�Ӧ�Ŀ����ȽǶ��о���Ӧ��2H2 + O2 = H2O�� ��֪�÷�ӦΪ���ȷ�Ӧ����ͼ����ȷ��ʾ�÷�Ӧ�������仯����________��
�Ӷϼ��ͳɼ��ĽǶȷ���������Ӧ�������ı仯����ѧ���ļ������±���
| ��ѧ�� | H��H | O��O | H��O |
| ����kJ/mol | 436 | 496 | 463 |
��2��ԭ��ؿɽ���ѧ��ת��Ϊ���ܡ���Fe��Cu��Ũ���ṹ��ԭ��أ������� ���Cu����Fe������ ��Zn��Ag��ϡ���ṹ��ԭ��أ��������� ��Ӧ�����������ԭ�������������Һ������������ �����������������������ͬ��ͭ����п���õ������Ӻ����CuSO4��Һ�У�һ��ʱ���ȡ��ϴ�����������������������Ϊ12.9 g��������ͨ���ĵ��ӵ����ʵ����� mol��
��3��һ���¶��£���3 molA�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)��B(g)
 xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ ��XΪ ������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ�� 0.8mol/L������ڣ�С�ڻ���ڡ���������֪��ƽ��ʱ���������ڻ��������ѹǿΪp�����������ʼѹǿΪp0������p0��p����ʾ��ƽ��ʱ��Ӧ��A��ת����Ϊ ��
xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ ��XΪ ������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ�� 0.8mol/L������ڣ�С�ڻ���ڡ���������֪��ƽ��ʱ���������ڻ��������ѹǿΪp�����������ʼѹǿΪp0������p0��p����ʾ��ƽ��ʱ��Ӧ��A��ת����Ϊ �� ��14�֣������ѣ�CH3OCH3���ͼ״���CH3OH�����Ǹ�Ч�����Դ����ҵ������ú���������ˮú�����ϳɼ״��Ͷ����ѡ��ش��������⣺
��1���Ʊ����������һ����Ӧ��Al2O3���״���ˮ�ϳɣ���Ӧ����ʽΪ ��
��2����֪��CO(g)+2H2(g)=CH3OH (g) ��H= ��90.1kJ��mol-1 CO(g)��ȼ������282.8 kJ��mol-1��H2��ȼ������285.8 kJ��mol-1д����ʾCH3OH (g) ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ ��
��3��������ֱ��ȼ�ϵ�رȼ״�ֱ��ȼ�ϵ�ظ���Ч���������Ķ����Ѻͼ״���ȫ�ŵ�ת�Ƶ��ӵ����ʵ���֮���� ���ö�����ֱ��ȼ�ϵ�ص����������ʳ��ˮ��������9.2g������ʱ������������������������Ϊ L��������£�
��4���ںϳ��а���ˮú��������Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±���
CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±���
| �¶� | 260�� | 280�� | 295�� | 310�� |
| COת���� | 89% | 80% | 75% | 60% |
����ʽ����280��ʱƽ�ⳣ�� ��
����ƽ����ϵ�У����H2��ѹǿռ��ѹ��30%��Ҫʹ��ϵ��COת���ʴﵽ70%��Ӧ��ʹ�¶� ������ߡ��������͡��������䡱��
������ȼ��ʱ�ܷų��������ȣ���Ҳ��Һ��ʯ��������Ҫ�ɷ֣���Ϊ��ԴӦ�������ǵ��ճ������������֪��
��2C3H8(g)��7O2(g) =6CO(g)��8H2O(g) ��H =��2389.8 kJ/mol
��2CO(g) + O2(g) =2CO2(g) ��H =��566 kJ/mol
��H2O(l) = H2O(g) ��H ="+" 44.0 kJ/mol
��1��д��C3H8ȼ��ʱȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��C3H8�ڲ�������������ȼ�գ�����CO��CO2��H2O(g)�������еIJ���ͨ��һ������̶����ܱ������У���һ�������·������¿��淴Ӧ��CO(g) +H2O(g)  CO2(g) +H2(g)�÷�Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���±���
CO2(g) +H2(g)�÷�Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���±���
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
| | H2O | CO | CO2 | H2 |
| �� ������/g�� | 1.8 | 8.4 | a | 1 |
| �� ������/g�� | 1.8 | 2.8 | 0 | 0 |
����ͼ��ʾ�����������з�Ӧ��t1ʱ�̴ﵽƽ�⣬��t2ʱ����ı�ijһ�������������仯���������t2ʱ�̸ı������������ �� ��������Ҫ�㼴�ɣ���

��3��CO2����NaOH��Һ���յõ�Na2CO3��NaHCO3��
�� Na2CO3��Һ������Ũ���ɴ�С��˳��Ϊ���� ��������
�� ��֪25��ʱ��H2CO3�ĵ���ƽ�ⳣ��K1 = 4.4��10-7 mol/L��K2 = 4.7��10-11 mol/L����Na2CO3��Һ��pHΪ11ʱ����Һ��c(HCO3-)��c(CO32-) = ��
�� 0.1 mol/L Na2CO3��Һ��c(OH-) -c(H+) = [�ú�c(HCO3��)��c(H2CO3)�ķ��ű�ʾ]��

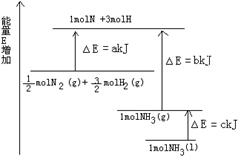
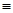 N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų� kJ������
N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų� kJ������
 O2(g)===
O2(g)=== P4O10(s) ��H2����738.5 kJ��mol��1
P4O10(s) ��H2����738.5 kJ��mol��1
 ���õ������������ݣ�
���õ������������ݣ�