��Ŀ����
��9�֣�����10��Ԫ�ص����ʡ��������±����У����Ǿ�Ϊ������Ԫ�ء��ش��������⣺
��1��H��Ԫ�ط����� ; B��Ԫ�����ڱ��е�λ���ǣ����ڡ��壩
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����ķ���ʽ�� ��
��3��������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ________________������Ӽ������Լ���Ǽ��Լ�����
��F2A2��J2A�ķ�Ӧ����3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2�������� g��
��4���õ���ʽ��ʾJ2A���γɹ��̣� ��
| | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶(10��9m) | 0.074 | 0.160 | 0.152 | 0.110 | 0.099 | 0.186 | 0.075 | 0.082 | 0.102 | 0.037 |
| ������ ���ϼ� | | ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | +6[ | +1 |
| ��2 | | | ��3 | ��1 | | ��3 | | -2 | |
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����ķ���ʽ�� ��
��3��������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ________________������Ӽ������Լ���Ǽ��Լ�����
��F2A2��J2A�ķ�Ӧ����3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2�������� g��
��4���õ���ʽ��ʾJ2A���γɹ��̣� ��
(1) B; �������ڵ�IIA�壻 ��2��HClO4;
(3)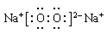 ���Ӽ����Ǽ��Լ���39g
���Ӽ����Ǽ��Լ���39g
��4��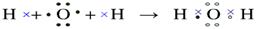
(�ڣ�4����2�֣�������ӷֱ��õ�Ͳ��ʾ�ˣ���ǰ��������Ӧ������û��)
��(3)��ڶ����ա����Ӽ����Ǽ��Լ���2�֣����һ����һ�֣�����ÿ��ÿ��1��
(3)
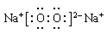 ���Ӽ����Ǽ��Լ���39g
���Ӽ����Ǽ��Լ���39g��4��
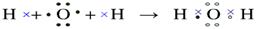
(�ڣ�4����2�֣�������ӷֱ��õ�Ͳ��ʾ�ˣ���ǰ��������Ӧ������û��)
��(3)��ڶ����ա����Ӽ����Ǽ��Լ���2�֣����һ����һ�֣�����ÿ��ÿ��1��
����Ԫ�������ɵ�Ӧ�á�ͬ������������ԭ�Ӱ뾶��С���������������ͬ�������϶���ԭ�Ӱ뾶�����ӷǽ���Ԫ�ؿ�ʼ���ָ��ۡ�Ԫ�ص���۾�������Ԫ�صļ۵��ӡ����Ը���Ԫ�ص���Ҫ���ϼۺ���Ҫ�뾶��֪��A��J�ֱ���O��Mg��Li��P��Cl��Na��N��B��S��H��������F2A2���ǹ������ƣ��������Ӽ��ͷǼ��Լ�������ʽΪ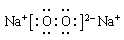 ��J2A��ˮ���������Ʒ�Ӧ�ķ���ʽΪ2Na2O2��2H2O=4NaOH��O2��,�ڷ�Ӧ�й������Ƽ���������Ҳ�ǻ�ԭ����ÿ����2mol�������ƣ���Ӧ�о�ת��2mol���ӡ�������3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2��������0.5mol��78g/mol��39g��ˮ���ɼ��Լ����ɵĹ��ۻ�������γɹ���Ϊ
��J2A��ˮ���������Ʒ�Ӧ�ķ���ʽΪ2Na2O2��2H2O=4NaOH��O2��,�ڷ�Ӧ�й������Ƽ���������Ҳ�ǻ�ԭ����ÿ����2mol�������ƣ���Ӧ�о�ת��2mol���ӡ�������3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2��������0.5mol��78g/mol��39g��ˮ���ɼ��Լ����ɵĹ��ۻ�������γɹ���Ϊ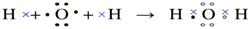
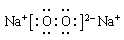 ��J2A��ˮ���������Ʒ�Ӧ�ķ���ʽΪ2Na2O2��2H2O=4NaOH��O2��,�ڷ�Ӧ�й������Ƽ���������Ҳ�ǻ�ԭ����ÿ����2mol�������ƣ���Ӧ�о�ת��2mol���ӡ�������3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2��������0.5mol��78g/mol��39g��ˮ���ɼ��Լ����ɵĹ��ۻ�������γɹ���Ϊ
��J2A��ˮ���������Ʒ�Ӧ�ķ���ʽΪ2Na2O2��2H2O=4NaOH��O2��,�ڷ�Ӧ�й������Ƽ���������Ҳ�ǻ�ԭ����ÿ����2mol�������ƣ���Ӧ�о�ת��2mol���ӡ�������3.01��1023������ת��ʱ���μӷ�Ӧ��F2A2��������0.5mol��78g/mol��39g��ˮ���ɼ��Լ����ɵĹ��ۻ�������γɹ���Ϊ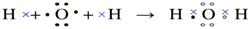

��ϰ��ϵ�д�
 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
�����Ŀ
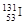 ��
��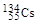 ��
��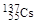 �������й�������ȷ����(����)
�������й�������ȷ����(����)