��Ŀ����
X��Y��Z��Q��WΪ��ԭ��������С�������е����ֶ�����Ԫ�ء���֪����X��Q����ͬһ���壬��ԭ�Ӽ۵����Ų�ʽ��Ϊn s2np2����Xԭ�Ӱ뾶С��Q����YԪ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺��·С��YԪ�أ���Wԭ�ӵļ۵����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ���ZԪ�صĵ��������ݼ��±���kJ��mol-1��
s2np2����Xԭ�Ӱ뾶С��Q����YԪ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺��·С��YԪ�أ���Wԭ�ӵļ۵����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ���ZԪ�صĵ��������ݼ��±���kJ��mol-1��
��ش�
��1��XY2���ӵĿռ乹��Ϊ ��
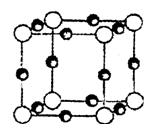
��2��QX�ľ���ṹ����ʯ�����ƣ�������ڵ��������� ��
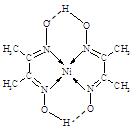
��3������ZW���۵�Ⱦ���XW4���Ըߵ�ԭ���� ��
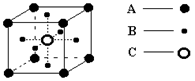
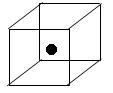
��4��XY2�ڸ��¸�ѹ�����γɵľ�����ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ���������©�壬�þ�����Xԭ�ӵ��ӻ���ʽΪ___ ��
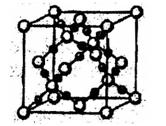
��5��������MO�ĵ���������QX����ȣ���MΪ ������Ԫ�ط��ţ���MO�����������²��ϣ��侧��ṹ��ZW����ṹ���ƣ�MO���۵��CaO�ĸߣ���ԭ���� ��
 s2np2����Xԭ�Ӱ뾶С��Q����YԪ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺��·С��YԪ�أ���Wԭ�ӵļ۵����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ���ZԪ�صĵ��������ݼ��±���kJ��mol-1��
s2np2����Xԭ�Ӱ뾶С��Q����YԪ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺��·С��YԪ�أ���Wԭ�ӵļ۵����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ���ZԪ�صĵ��������ݼ��±���kJ��mol-1��| I1 | I2 | I3 | I4 | �� |
| 496 | 4562 | 6912 | 9540 | �� |
��1��XY2���ӵĿռ乹��Ϊ ��
��2��QX�ľ���ṹ����ʯ�����ƣ�������ڵ��������� ��
��3������ZW���۵�Ⱦ���XW4���Ըߵ�ԭ���� ��
��4��XY2�ڸ��¸�ѹ�����γɵľ�����ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ���������©�壬�þ�����Xԭ�ӵ��ӻ���ʽΪ___ ��

��5��������MO�ĵ���������QX����ȣ���MΪ ������Ԫ�ط��ţ���MO�����������²��ϣ��侧��ṹ��ZW����ṹ���ƣ�MO���۵��CaO�ĸߣ���ԭ���� ��

��

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
 CH3CH2OH(g)+3H2O(g) ��H��a kJ��mol��1
CH3CH2OH(g)+3H2O(g) ��H��a kJ��mol��1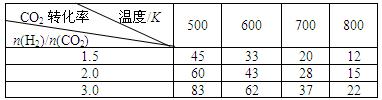
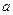 0(����ڡ���С�ڡ�)��
0(����ڡ���С�ڡ�)�� )�ȣ�ƽ�ⳣ��Kֵ �����������С�������䡱�����������Ҵ� �������������������
)�ȣ�ƽ�ⳣ��Kֵ �����������С�������䡱�����������Ҵ� �������������������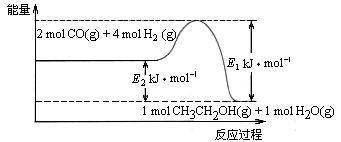

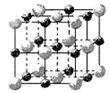

 ��ʾ��������B��
��ʾ��������B�� ��ʾ����ÿ�������к�A���ӵ���ĿΪ________����B������ĿΪ________����A�ĺ�������Ų���Ar��ͬ��B�ĺ�������Ų���Ne��ͬ��������ӻ�����Ļ�ѧʽ��___________________��
��ʾ����ÿ�������к�A���ӵ���ĿΪ________����B������ĿΪ________����A�ĺ�������Ų���Ar��ͬ��B�ĺ�������Ų���Ne��ͬ��������ӻ�����Ļ�ѧʽ��___________________��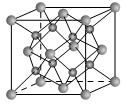
 ����B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
����B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��