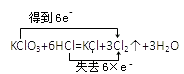
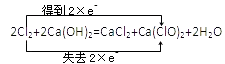��Ŀ����
����Ŀ����1������������Һ��pH��3��HA��ҺV1 mL��pH��11��NaOH��ҺV2 mL��϶��ã�������˵����ȷ����__________��
A������Ϻ���Һ�����ԣ�c(H��)��c(OH��)��2��10��7mol/L
B����V1��V2����Ϻ���ҺpHһ������7
C������Ϻ���Һ�����ԣ���V1һ������V2
D������Ϻ���Һ�ʼ��ԣ���V1һ��С��V2
��2�������£�Ũ�Ⱦ�Ϊ0.1 mol/L������������Һ��pH�����ʾ��
���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
�ٸ��ݱ������ݣ���Ũ�Ⱦ�Ϊ0.01 mol/L���������������Һ�ֱ�ϡ��100����pH�仯��С����__________��
A��HCN B��HClO C��H2CO3 D��CH3COOH
�ڸ����������ݣ��ж����з�Ӧ���Գ�������________��
A.CH3COOH �� Na2CO3 ==NaHCO3 �� CH3COONa
B.CH3COOH �� NaCN ==CH3COONa�� HCN
C.CO2 �� H2O�� 2 NaClO ==Na2CO3��2 HClO
D.NaHCO3 �� HCN == NaCN�� H2O�� CO2��
��3������������ˮ�õ���ˮ����һ����������£��ֽ�amol/L��ˮ��0.01mol/L����������Ϻ���Һ��c(NH4+)=c(Cl-)����ˮ��Kb= ____________�����ú�a�Ĵ���ʽ��ʾ��
��4��25������0.1000 mol / LNaOH��Һ�ζ�20��00mL0.1000mol/LijһԪ��HA��Һ���õζ�������ͼ��

��Ϊ��Сʵ������ͼ��֪�ζ�ʱָʾ��Ӧѡ��__________ ������ʯ����������̪����������������
��A��B��C������ʾ��Һ����������ǿ����_________���Ӧ����Һ��
�۱Ƚ�A��C������ˮ�ĵ���̶ȣ�A ______ C(������������=����������)��
���𰸡� AD A AB ![]() ��̪ C ��
��̪ C ��
��������(1).pH=3��HA��Һ����c(HA)0.001mol/L��pH=11��NaOH��Һ����c(NaOH)=0.001mol/L��
A. ����ҺM������,��c(H+)=c(OH)=1��107molL1����c(H+)+c(OH)=2��107molL1����A��ȷ��
B. ��V1=V2������HA��ǿ��δ֪����Ӧ����Һ������Բ���ȷ������ҺM��pH��һ������7����B����
C. ��HAΪ������������������ҺҲ���ܳ���������V1��һ������V2����C����
D. ��HAΪ�������������ϳ���������ʼ�����V1һ��С��V2����HAΪǿ������Ӧ��ʼ�������V1һ��С��V2����D��ȷ��
�ʴ�Ϊ��AD��
(2). ��.��ͬŨ�ȵ�����Һ���������Խ�������������ˮ��̶�Խ��������������ҺpHȷ�����ǿ������ͬŨ�ȵIJ�ͬ������ˮϡ�ʹٽ������������ϡ����ͬ�ı������������Խ������Һϡ������pH�仯ԽС������������Һ��pH֪��HCN��HClO��CH3COOH��H2CO3����������Դ�С˳����CH3COOH>H2CO3>HClO>HCN��������Һ��pH�仯��С����HCN����ѡA��
��. . HCN��HClO��CH3COOH��H2CO3����������Դ�С˳����CH3COOH>H2CO3>HClO>HCN������ǿ����ȡ����֪��
A. ����CH3COOH>H2CO3>HCO3�������Զ��߷�ӦΪCH3COOH �� Na2CO3 ==NaHCO3 �� CH3COONa����A��ȷ��
B. �������Դ���HCN�����Զ��߷�ӦΪCH3COOH �� NaCN ==CH3COONa�� HCN����B��ȷ��
C. ̼�����Դ��ڴ����������Զ��߷�ӦΪCO2+H2O+NaClO�TNaHCO3+NaClO����C����
D. ����H2CO3>HClO>HCN������NaHCO3��HCN���߲���Ӧ����D����
��ѡAB��
(3). д������غ�ʽ��c(NH4+)+c(H+)=c(Cl)+c(OH)����c(NH4+)=c(Cl)����c(H+)=c(OH)����Һ�����ԣ��ٸ��ݳ�����Kw=10��14����c(H+)=c(OH)=107mol/L������ˮ�еĵ��볣��ΪKb= c(NH4+)��c(H+)/ c(NH3��H2O)=0.01��107/(a��0.01)= ![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ![]() ��
��
(4). ��.��ͼ��֪������ǡ����ȫ��Ӧʱ����Һ�ʼ��ԣ�Ӧѡ����Ա�ɫ��Χ�ڵ�ָʾ��������ѡ��̪��
��.C��ʱ����Ũ�����������������ǿ�����Դ���C�㣻
��.����NaOH�ĵ��룬HAԽ��Խ�٣����ˮ�ĵ�������Խ��Խ����ˮ�ĵ���̶�Խ��Խ��C��ʱ��ǡ����ȫ��Ӧ����Cʱˮ�ĵ���̶���ʴ��ǣ�����

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�