��Ŀ����
�����裨Si3N4����һ�������ĸ��½ṹ�մɣ��ڹ�ҵ�����ͿƼ���������Ҫ��;��
I����ҵ���ж��ַ������Ʊ��������裬�����ķ����У�
����һ ֱ�Ӻ���������1300~1400��ʱ���ߴ���״���봿�������ϣ��䷴Ӧ����ʽΪ
������ ��ѧ������������ڸ����������������Ȼ������塢��������������Ӧ���ɺ������HCl���뷽��һ��ȣ��ô˷��Ƶõĺ����贿�Ƚϸߣ���ԭ���� ��
������ Si��NH2��4�ȷֽⷨ���������Ȼ����백����Ӧ����Si��NH2��4��һ������
�������ʽ����Ȼ��ʹSi��NH2��4���ȷֽ⣬�ֽ�����һ�ֲ���ķ���ʽΪ ��
II����1�������迹��ʴ������ǿ�����ױ�����ḯʴ��������������ᷴӦ�����ķ������һ����Σ������д��ڵĻ�ѧ�������� ��
��2����֪��25�棬101kPa�����µ��Ȼ�ѧ����ʽ��
3Si��s��+2N2��g��==Si3N4��s�� ��H=��750.2kJ/mol
Si��s��+2Cl2��g��==SiCl4��g�� ��H=��609.6kJ/mol
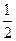 H2��g��+
H2��g��+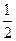 Cl2��g��==HCl��g�� ��H=��92.3kJ/mol
Cl2��g��==HCl��g�� ��H=��92.3kJ/mol
��д�����Ȼ��������뵪����������Ӧ���Ȼ�ѧ����ʽ��
��
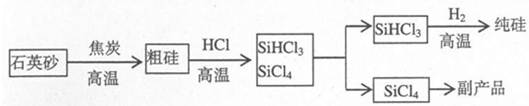
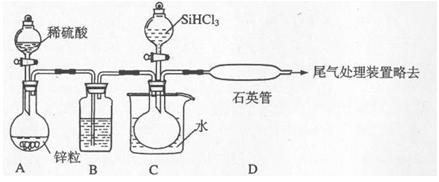
III����ҵ����ȡ�ߴ�������Ȼ���������������£�
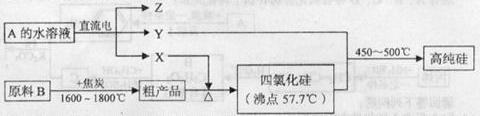
��֪��X���ߴ��衢ԭ��B����Ҫ�ɷֶ�����Z��Ӧ��Y��X�ڹ��ջ��ȼ�����¿ɷ�Ӧ��Z����ɫ�ʻ�ɫ��
��1��ԭ��B����Ҫ�ɷ��� ��
��2��д����̿��ԭ��B�е���Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽ�� ��
��3���������������е��A��ˮ��Һʱ�����������ܷ���Cu�� ����ܡ����ܡ�����
д��CuΪ�������A��ˮ��Һ��ʼһ��ʱ���������ĵ缫����ʽ��
������ �������� ��
I����ҵ���ж��ַ������Ʊ��������裬�����ķ����У�
����һ ֱ�Ӻ���������1300~1400��ʱ���ߴ���״���봿�������ϣ��䷴Ӧ����ʽΪ
������ ��ѧ������������ڸ����������������Ȼ������塢��������������Ӧ���ɺ������HCl���뷽��һ��ȣ��ô˷��Ƶõĺ����贿�Ƚϸߣ���ԭ���� ��
������ Si��NH2��4�ȷֽⷨ���������Ȼ����백����Ӧ����Si��NH2��4��һ������
�������ʽ����Ȼ��ʹSi��NH2��4���ȷֽ⣬�ֽ�����һ�ֲ���ķ���ʽΪ ��
II����1�������迹��ʴ������ǿ�����ױ�����ḯʴ��������������ᷴӦ�����ķ������һ����Σ������д��ڵĻ�ѧ�������� ��
��2����֪��25�棬101kPa�����µ��Ȼ�ѧ����ʽ��
3Si��s��+2N2��g��==Si3N4��s�� ��H=��750.2kJ/mol
Si��s��+2Cl2��g��==SiCl4��g�� ��H=��609.6kJ/mol
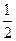 H2��g��+
H2��g��+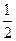 Cl2��g��==HCl��g�� ��H=��92.3kJ/mol
Cl2��g��==HCl��g�� ��H=��92.3kJ/mol��д�����Ȼ��������뵪����������Ӧ���Ȼ�ѧ����ʽ��
��
III����ҵ����ȡ�ߴ�������Ȼ���������������£�

��֪��X���ߴ��衢ԭ��B����Ҫ�ɷֶ�����Z��Ӧ��Y��X�ڹ��ջ��ȼ�����¿ɷ�Ӧ��Z����ɫ�ʻ�ɫ��
��1��ԭ��B����Ҫ�ɷ��� ��
��2��д����̿��ԭ��B�е���Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽ�� ��
��3���������������е��A��ˮ��Һʱ�����������ܷ���Cu�� ����ܡ����ܡ�����
д��CuΪ�������A��ˮ��Һ��ʼһ��ʱ���������ĵ缫����ʽ��
������ �������� ��

��

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
 4.7��10��8��д����84����Һ��(��Ҫ�ɷ�Ϊ�Ȼ��ƺʹ�������)��ͨ�������̼������Ӧ�����ӷ���ʽ ��
4.7��10��8��д����84����Һ��(��Ҫ�ɷ�Ϊ�Ȼ��ƺʹ�������)��ͨ�������̼������Ӧ�����ӷ���ʽ ��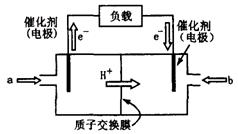
 ��ͼ������ܷ�ӦΪ2CH3OH��3O2
��ͼ������ܷ�ӦΪ2CH3OH��3O2 2CO2��4H2O����װ�÷ŵ�ʱ ���a����b����Ϊ��صĸ�������缫��ӦʽΪ ��
2CO2��4H2O����װ�÷ŵ�ʱ ���a����b����Ϊ��صĸ�������缫��ӦʽΪ ��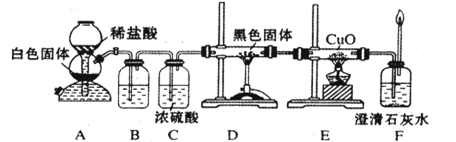
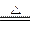 CO+H2 CO + H2O
CO+H2 CO + H2O H2 C + CO2
H2 C + CO2 2CO
2CO 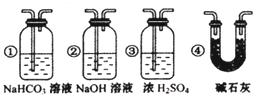
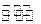 SiHCl3��g��+H2��g��
SiHCl3��g��+H2��g�� 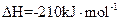
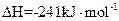
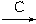 Si���ֹ裩
Si���ֹ裩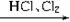 SiHCl3���е�31��5�棩
SiHCl3���е�31��5�棩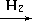 Si
Si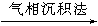 �����裨�ྦྷ�裩
�����裨�ྦྷ�裩 ������
������ ��������������
��������������