��Ŀ����
��֪п��ϡ���ᷴӦΪ���ȷ�Ӧ��ijѧ��Ϊ��̽���䷴Ӧ�����е����ʱ仯������ˮ�������ռ���Ӧ�ų���������ʵ���¼���£�
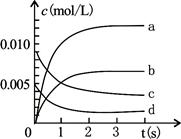
��Ӧ�������ģ���0��1��1��2��2��3��3��4��4��5min��ʱ���Ϊ ��ԭ���� ��
�ڷ�Ӧ������С��ʱ���Ϊ ��ԭ����
��2����һѧ��Ҳ��ͬ����ʵ�飬���ڷ�Ӧ̫�죬�������������ʱ���ÿ��ƣ�����������������Һ�зֱ����������������Һ�Լ�����Ӧ���ʡ�
A.����ˮ B.CuCl2��Һ C.NaCl��Һ
����Ϊ�����������е��� ������Ӧ��ĸ�������������е��� ������Ӧ��ĸ�������������е������� ��
| ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
| ���������mL�� | 30 | 120 | 280 | 350 | 370 |
��Ӧ�������ģ���0��1��1��2��2��3��3��4��4��5min��ʱ���Ϊ ��ԭ���� ��
�ڷ�Ӧ������С��ʱ���Ϊ ��ԭ����
��2����һѧ��Ҳ��ͬ����ʵ�飬���ڷ�Ӧ̫�죬�������������ʱ���ÿ��ƣ�����������������Һ�зֱ����������������Һ�Լ�����Ӧ���ʡ�
A.����ˮ B.CuCl2��Һ C.NaCl��Һ
����Ϊ�����������е��� ������Ӧ��ĸ�������������е��� ������Ӧ��ĸ�������������е������� ��
��1����2��3min �÷�Ӧ�Ƿ��ȷ�Ӧ����ʱ�¶ȸߣ�H+Ũ�ȴ�
��4��5min ��ʱH+Ũ��С
��2��A��C�� B�� Zn �û���Cu���γ�Cu��Znԭ��أ�ʹ��Ӧ����
��4��5min ��ʱH+Ũ��С
��2��A��C�� B�� Zn �û���Cu���γ�Cu��Znԭ��أ�ʹ��Ӧ����
����������¶�Խ�ߣ���Ӧ����Խ�죻����Ũ��ԽС����Ӧ����Խ�����������Ӵ��������Ӧ���ʼӿ죬������Һ�м���������NaCl��Һ���൱�ڼ�ˮ������ϡ�ͣ�����������Ũ�ȼ�С���ݴ˿��Խ��
��1���ٸ��ݱ������ݿ�֪��0��1��l��2��2��3��3��4��4��5min��ʱ�����������������ֱ�Ϊ30��90��160��70��20�����Կ�����Ӧ����������2��3min����������п��ϡ���ᷴӦΪ���ȷ�Ӧ���淴Ӧ�Ľ��У��¶�Խ��Խ�ߣ���Ӧ���ʼӿ졣
�ڷ�Ӧ������С��ʱ���Ϊ4��5min�������������ŷ�Ӧ�Ľ��У�������Ũ��Խ��ԽС����Ӧ����Խ��Խ����
��2��ѡ��A����������Һ�м�������������ˮ���൱�ڼ�ˮ������ϡ�ͣ�����������Ũ�ȼ�С����Ӧ���ʼ�������A��ȷ��ѡ��B�м����Ȼ�ͭ��Һ��п�û���ͭ���γ�Cu��Znԭ��أ�ʹ��Ӧ���ʸ��죬B����ȷ��ѡ��C��������������Һ�м���������NaCl��Һ���൱�ڼ�ˮ������ϡ�ͣ�����������Ũ�ȼ�С����Ӧ���ʼ�������C��ȷ��
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������ͣ�����������ѧ���淶�Ͻ���ʵ���������������������ѧ����ѧ�����������ѧ����Ӧ������������������Ҫ����ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ
 2CO(g) ��H��0���ﵽƽ��״̬���ֽ������²����������߷�Ӧ��ϵ���¶ȣ������ӷ�Ӧ��C������������С��Ӧ��ϵ��������ܼ�С��ϵ��CO������������ʩ��һ����ʹ��Ӧ������Ӧ���������ӿ���� �� ��
2CO(g) ��H��0���ﵽƽ��״̬���ֽ������²����������߷�Ӧ��ϵ���¶ȣ������ӷ�Ӧ��C������������С��Ӧ��ϵ��������ܼ�С��ϵ��CO������������ʩ��һ����ʹ��Ӧ������Ӧ���������ӿ���� �� ��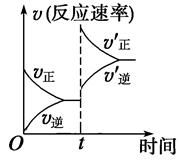


 2NH3����H<0�����ʡ�ʱ��ͼ����t1ʱ��ʹƽ�ⷢ���ƶ���ԭ����
2NH3����H<0�����ʡ�ʱ��ͼ����t1ʱ��ʹƽ�ⷢ���ƶ���ԭ����
 2NO2(g)������Ӧ���ȡ����n(NO)��ʱ��ı仯���±�����ش�
2NO2(g)������Ӧ���ȡ����n(NO)��ʱ��ı仯���±�����ش�