��Ŀ����
A��B��C��D 4��1~20��Ԫ�أ�AԪ��������������������������ԭ����������ȣ�B��ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ������2����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��
(1) B�����ڱ��е�λ�õ� ______���ڣ���________�壻
(2) A��B�γɵĻ�����Ľṹʽ____ ____�����к��еĻ�ѧ������Ϊ________��
�õ���ʽ��ʾ�û������γɹ��� ___ _��
(3) C��Ԫ�ط���________��������ӵĵ���ʽ____ ____��
(4) д��D��A�γɻ�����ĵ���ʽ____ ____��
���𰸡�
��1��2����A����2�֣� ��2����2�֣������Լ���1�֣���
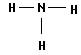
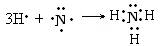
��3��S��SO3��2�֣� ��4��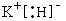 (2��)
(2��)

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ






