��Ŀ����
16����������ϡ�Ȼ�������Һ�м���1��2��Һ�壬����Һ��Ϊ��ɫ����1����ͬѧ��Ϊ��Br2����ˮ��ɻ�ɫ��Һ�� ����Ϊ��Fe2+��������Fe3+ʹ��Һ��ɻ�ɫ��
��2�����ṩ�Լ���
A�����Ը��������Һ B������������Һ C�����Ȼ�̼ D�����軯����Һ
���ж��ң���ס����ҡ������ƶ���ȷ���������ַ���������֤��д��ѡ�õ��Լ���ż�ʵ���й۲쵽������
| ѡ���Լ� | ʵ������ | |
| ��һ�ַ��� | C | �л�����ɫ |
| �ڶ��ַ��� | D | ��Һ��� |
���� ��2������Ϣ��֪����ҺΪ��ɫ��ԭ�������֣����嵥�ʻ��������ӱ����������������ӣ�ʵ��֤����ͬѧ���ƶ�����ȷ�ģ�֤�������嵥�ʻ���������Ӿ��ɣ�
��3��������������֪����ԭ���������Ӵ��������ӣ���ϡ�廯������Һ��ͨ����������ԭ��ǿ���ױ�������
��� �⣺��2������Ϣ��֪����ҺΪ��ɫ��ԭ�������֣����嵥�ʻ��������ӱ����������������ӣ������Ϊ��Һ���ɫ��������Br2������Ϊ��Һ���ɫ��������Fe3+��FeCl3��FeBr3����ʵ��֤����ͬѧ���ƶ�����ȷ�ģ�֤�������嵥�ʻ���������Ӿ���
��ѡ���Ȼ�̼ʱ�л���Ϊ��ɫ�����嵥�ʣ�˵����ͬѧ��ȷ��
ѡNaOH��Һ�����ֺ��ɫ������˵����ͬѧ��ȷ��
ѡ���軯�أ���Һ��Ϊ��ɫ��˵����ͬѧ��ȷ��
�ʴ�Ϊ���ң�
| ѡ���Լ� | ʵ������ | |
| ��һ�ַ��� | C | �л�����ɫ |
| �ڶ��ַ��� | D | ��Һ��� |
��3��������������֪����ԭ���������Ӵ��������ӣ���ϡ�廯������Һ��ͨ����������ԭ��ǿ���ױ���������Fe2+�ȱ�����������ϡ�廯������Һ��ͨ����������ʱ���������Ӿ������������ӷ�ӦΪ2Fe2++4Br-+Cl2=2Fe3++2Br2+6Cl-��Br2��Fe3+���ܰ�I-������I2�����Բ����õ��۵⻯����Һ���飬
�ʴ�Ϊ�������У���ΪBr2��Fe3+���ܰ�I-������I2��
���� ���⿼������ʵ�鷽������ƣ���ȷ���ʵ������ǽⱾ��ؼ����漰���ӵļ��顢������ԭ��Ӧ��֪ʶ�㣬�������ʷ�Ӧ��������ԭ��ǿ��˳���֪ʶ�����������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
4�������£�������Һ����Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | Na2S ��Һ��c�� Na+ ����c��HS- ����c�� OH- ����c��H2S�� | |
| B�� | Na2C2O4 ��Һ��c �� OH- ��=c �� H+ ��+c��HC2O4-��+2c��H2C2O4�� | |
| C�� | Na2CO3��Һ��c��Na+��+c��H+��=2c��CO32-��+c��OH-�� | |
| D�� | CH3COONa �� CaCl2 �����Һ��c��Na+ ��+c��Ca 2+ ���Tc��CH3COO- ��+c��CH3COOH��+2c��Cl- �� |
11���廯����һ����Ҫ�Ļ���ԭ�ϣ��Ʊ�CaBr2•2H2O ����Ҫ������ͼ������˵������ȷ���ǣ�������


| A�� | �Լ�M ��HBr������HBr��Ŀ���dz�ȥ������Ca��OH��2 | |
| B�� | ����IΪ���ˣ�����ΪFe��Fe��OH��2��Fe��OH��3��������Ϊ�ؽᾧ�������������Ϊ����Ũ������ȴ��Ʒ��ϴ�ӡ����� | |
| C�� | �Ƶõ�CaBr2•2H2O ����ͨ�����·����ⶨ���ȣ���ȡһ��������Ʒ����ˮ����������Na2CO3��Һ����ַ�Ӧ����ˣ�������ϴ�ӡ���ɡ���ȴ�������������ó�CaBr2•2H2O�Ĵ��� | |
| D�� | ��ҵ��Ҳ���Խ�����ͨ��ʯ���飬�������壬��65����з�Ӧ���Ƶ��廯�ƣ��˷�Ӧ�л�������һ����ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ3Ca��OH��2+3Br2+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3CaBr2+N2+6H2O |
8�������й�˵����ȷ���ǣ�������
| A�� | ��ӦCH3Cl��g��+Cl2��g��$\stackrel{����}{��}$CH2Cl2��g��+HCl��g�� ���Է����У��÷�Ӧ�ġ�H��0 | |
| B�� | �����ԭ���У�1s��2s��3s���ӵ�����������2p��3p��4p�ܼ��Ĺ������������ | |
| C�� | �� +H2$\stackrel{����}{��}$ +H2$\stackrel{����}{��}$ ��H=+23kJ•mol-1��֪�� ��H=+23kJ•mol-1��֪�� �� �� ���ȶ� ���ȶ� | |
| D�� | ���������ˮ�ⷴӦ������ϡ�����ʹƽ��������Ӧ�����ƶ� |
6�����й������ʵķ��롢�ᴿʵ���е�һЩ��������������ȷ���ǣ�������
| A�� | ���Ȼ�̼�������Ҵ�����������ȡ������Ȼɫ�� | |
| B�� | ��96%�Ĺ�ҵ�ƾ���ȡ��ˮ�Ҵ����ɲ��õķ����Ǽ���ʯ�ң������� | |
| C�� | �ڱ������ؽᾧ��ʵ���У�������ͣ������ֽ�� | |
| D�� | �ڱ������ؽᾧ��ʵ���У��ֱ�������ȫ�ܽ��Ҫ��ȴ�����²Ź��� |


 ��
�� ��D��E��Ӧ�����Ǽӳɷ�Ӧ��
��D��E��Ӧ�����Ǽӳɷ�Ӧ�� ��
�� ��
�� ��
�� ��
�� =O+RMgX��
=O+RMgX�� $\stackrel{H_{2}O}{��}$
$\stackrel{H_{2}O}{��}$ ������ϩ�ͱ�Ҫ������Ϊԭ�Ϻϳ�2-�����������ϳ�һ�ַ���ʽΪC6H8O4�ľ�����Ԫ��������J���ϳ���·��ͼ��G�������ֲ�ͬ��ѧ��������ԭ�ӣ�
������ϩ�ͱ�Ҫ������Ϊԭ�Ϻϳ�2-�����������ϳ�һ�ַ���ʽΪC6H8O4�ľ�����Ԫ��������J���ϳ���·��ͼ��G�������ֲ�ͬ��ѧ��������ԭ�ӣ�
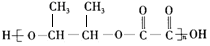 ��
��