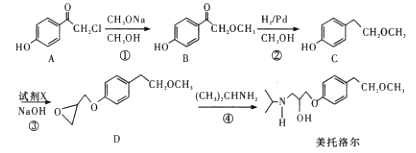
̀âÄ¿ÄÚÈƯ
¡¾̀âÄ¿¡¿Ä³»¯Ñ§Đ¡×é²ÉÓĂÀàËÆÖÆ̉̉Ëá̉̉ơ¥µÄ×°ÖĂ(Èçͼ)£¬̉Ô»·¼º´¼ÖƱ¸»·¼ºÏ©


(1)ÖƱ¸´ÖÆ·
½«12.5mL»·¼º´¼¼ÓÈëÊÔ¹ÜAÖĐ£¬ÔÙ¼ÓÈëlmLŨẠ́Ëᣬ̉¡ÔȺó·ÅÈëËé´ÉƬ£¬»ºÂư¼ÓÈÈÖÁ·´Ó¦ÍêÈ«£¬ÔÚÊÔ¹ÜCÄڵõ½»·¼ºÏ©´ÖÆ·¡£
¢ÙAÖĐËé´ÉƬµÄ×÷ÓĂÊÇ___________£¬µ¼¹ÜB³ưÁ˵¼ÆøÍ⻹¾ßÓеÄ×÷ÓĂÊÇ___________¡£
¢ÚÊÔ¹ÜCÖĂÓÚ±ùˮԡÖеÄÄ¿µÄÊÇ____________________________________________¡£
(2)ÖƱ¸¾«Æ·

¢Ù»·¼ºÏ©´ÖÆ·ÖĐº¬ÓĐ»·¼º´¼ºÍÉÙÁ¿ËáĐÔÔÓÖʵȡ£¼ÓÈë±¥ºÍʳÑÎË®£¬Ơñµ´¡¢¾²ÖĂ¡¢·Ö²ă£¬»·¼ºÏ©ÔÚ_________²ă(̀îÉÏ»̣ÏÂ)£¬·Ö̉ººóÓĂ_________ (̀îÈë±àºÅ)Ï´µÓ¡£
a£®KMnO4ÈÜ̉º b£®Ï¡H2SO4 c£®Na2CO3ÈÜ̉º
¢ÚÔÙ½«»·¼ºÏ©°´ÈçͼװÖĂƠôÁó£¬ƠôÁóʱ̉ª¼ÓÈëÉúʯ»̉£¬Ä¿µÄÊÇ__________________¡£
¢ÛÊƠ¼¯²úƷʱ£¬¿ØÖƵÄζÈÓ¦ÔÚ_________×óÓ̉£¬ÊµÑéÖÆµĂµÄ»·¼ºÏ©¾«Æ·ÖÊÁ¿µÍÓÚÀíÂÛ²úÁ¿£¬¿ÉÄܵÄỘ̉ÊÇ£¨______£©
a£®ƠôÁóʱ´Ó70¡æ¿ªÊ¼ÊƠ¼¯²úÆ·
b£®»·¼º´¼Êµ¼ÊÓĂÁ¿¶àÁË
c£®ÖƱ¸´ÖƷʱ»·¼º´¼Ëæ²úÆ·̉»ÆđƠô³ö
(3)̉ÔÏÂÇø·Ö»·¼ºÏ©¾«Æ·ºÍ´ÖÆ·µÄ·½·¨£¬ºÏÀíµÄÊÇ_________¡£
a£®ÓĂËáĐÔ¸ßẰËá¼ØÈÜ̉º b£®ÓĂ½đÊôÄÆ c£®²â¶¨·Đµă
(4)Éè¼ÆʵÑé¼́Ñé»·¼ºÏ©¾«Æ·ÖĐÊÇ·ñº¬¼º¶₫È©_______________________¡£
¡¾´đ°¸¡¿·À±©·Đ ÀäÄư ·ÀÖ¹»·¼ºÏ©»Ó·¢ ÉÏ c ¸ÉÔï 83¡æ c b È¡¾«Æ·ÊỔºÉÙĐíÓÚÊÔ¹ÜÖĐ,¼ÓÈë̉ø°±ÈÜ̉ºĐÂÖÆÇâÑơ»¯ÍĐü×Ç̉ººó¼ÓÈÈ,ÈôÄܹ۲́µ½×©º́É«µÄ³Áµí,ỘÖ¤Ă÷»·¼ºÏ©¾«Æ·ÖĐº¬ÓĐ¼º¶₫È©,·´Ö®ỘÎ̃(´đ°¸ºÏÀí¾ù¸ø·Ö)
¡¾½âÎö¡¿
»·¼º´¼ÔÚŨẠ́Ëá´æÔÚϼÓÈȵ½85¶ÈÉú³É»·¼ºÏ©£¬Ó¦²ÉÓĂˮԡ¼ÓÈÈ£¬³¤µ¼¹Ü¿É̉ÔÆäÀäÄưµÄ×÷ÓĂ¡£·ÖÀë»·¼ºÏ©ÖеĻ·¼º´¼ºÍËáĐÔÔÓÖÊ£¬Đè̉ª½øĐĐ·Ö̉º£¬È»ºóÓẰ¼ËáÄÆÈÜ̉ºÏ´µÓ£¬¼ơÉÙ²úÆ·ÖеĻ·¼º´¼ºÍËáĐÔÔÓÖÊ£¬»·¼º´¼ÄܺͽđÊôÄÆ·´Ó¦£¬µ«»·¼ºÏ©²»ÄÜ¡£¶₫Ơ߶¼ÄܺÍËáĐÔ¸ßẰËá¼ØÈÜ̉º·´Ó¦¡£
(1) ¢Ù̉º̀åÖĐ¼ÓÈëËé´ÉƬµÄ×÷ÓĂÊÇ·ÀÖ¹̉º̀å¼ÓÈȹư³̀ÖĐ¾çÁ̉·Đ̀Ú¡£³¤µ¼¹ÜÓе¼³öÆø̀åºÍÀäÄưµÄ×÷ÓĂ¡£
¢Ú»·¼ºÏ©µÄ·Đµă½ÏµÍ£¬Îª83¡æ£¬·ÅÔÚ±ùˮԡÖĐ¿É̉Ô·ÀÖ¹»·¼ºÏ©»Ó·¢£»
(2) ¢Ù»·̉̉Ï©µÄĂܶȱÈË®Đ¡£¬ÔÚÉÏ²ă£¬·Ö̉ººóÓẰ¼ËáÄÆÈÜ̉ºÏ´µÓ£¬Ï´È¥ËáĐÔÔÓÖʺͻ·¼º´¼µÈ£»
¢ÚÉúʯ»̉ÄܺÍË®·´Ó¦£¬Ëù̉ÔƠôÁóʱ¼ÓÈëÉúʯ»̉ÄܸÉÔ
¢Ụ̂̉Ϊ»·¼ºÏ©µÄ·ĐµăΪ83¡æ£¬Ëù̉Ô¿ØÖƵÄζÈÓ¦ÔÚ83¡æ×óÓ̉£»Èôa£®ƠôÁóʱ´Ó70¡æ¿ªÊ¼ÊƠ¼¯²úÆ·£¬Ộ²úÆ·µÄÖÊÁ¿¸ß£¬¹Ê´íÎó£»b£®»·¼º´¼Êµ¼ÊÓĂÁ¿¶àÁË£¬»áʹ²úÆ·µÄÁ¿Ôö¼Ó£¬¹Ê´íÎó£»c£®ÖƱ¸´ÖƷʱ»·¼º´¼Ëæ²úÆ·̉»ÆđƠô³ö£¬»áʹÉú³ÉµÄ»·̉̉Ï©Á¿¼ơÉÙ£¬¹ÊƠưÈ·¡£¹ÊÑ¡c¡£
(3)̣̉Ϊ»·¼ºÏ©ºÍ»·¼º´¼¶¼ÄÜʹËáĐÔ ¸ßẰËá¼ØÈÜ̉ºÍÊÉ«£¬¶ø»·¼º´¼ÄÜÓë½đÊôÄÆ·´Ó¦£¬µ«»·¼ºÏ©²»ÄÜ£¬²â¶¨·ĐµăµÄ·½·¨²»ÄÜʵÏÖ£¬¹ÊºÏÀíµÄ·½·¨Îªb¡£
(4) ÀûÓĂÈ©»ùÄÜ·¢Éú̉ø¾µ·´Ó¦»̣ÓëĐÂÖƵÄÇâÑơ»¯Í·´Ó¦Éú³Éº́É«³Áµí½øĐĐ¼́Ñ飬·½·¨Îª£ºÈ¡¾«Æ·ÊỔºÉÙĐíÓÚÊÔ¹ÜÖĐ£¬¼ÓÈë̉ø°±ÈÜ̉ºĐÂÖÆÇâÑơ»¯ÍĐü×Ç̉ººó¼ÓÈÈ,ÈôÄܹ۲́µ½×©º́É«µÄ³Áµí£¬ỘÖ¤Ă÷»·¼ºÏ©¾«Æ·ÖĐº¬ÓĐ¼º¶₫È©£¬·´Ö®ỘÎ̃¡£

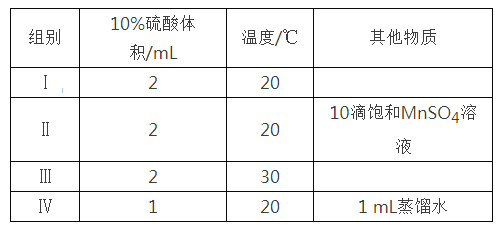
¡¾̀âÄ¿¡¿£¨1£©25 ¡æʱ£¬ÖƱ¸ÑÇÏơơ£ÂÈËùÉæ¼°µÄÈÈ»¯Ñ§·½³̀ʽºÍƽºâ³£ÊưÈç±í£º
ÈÈ»¯Ñ§·½³̀ʽ | ƽºâ³£Êư | |
¢Ù | 2NO2(g)+NaCl(s) | K1 |
¢Ú | 4NO2(g)+2NaCl(s) | K2 |
¢Û | 2NO(g)+Cl2(g) | K3 |
Ộ¸ĂζÈÏ£¬¦¤H3=_______________kJmol-1£»K3=_____________£¨ÓĂK1ºÍK2±íʾ£©¡£
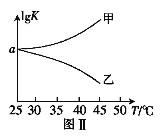
£¨2£©25¡æʱ£¬ÔÚ̀å»ưΪ2LµÄºăÈƯĂܱƠÈƯÆ÷ÖĐͨÈë0.08 mol NOºÍ0.04 molCl2·¢ÉúÉÏÊö·´Ó¦¢Û£¬Èô·´Ó¦¿ªÊ¼Óë½áÊøʱζÈÏàͬ£¬Êư×Öѹǿ̉ÇÏÔʾ·´Ó¦¹ư³̀ÖĐѹǿ(p)Ëæʱ¼ä(t)µÄ±ä»¯Èçͼ¢ñʵÏßËùʾ£¬Ộ¦¤H3 ___£῭î¡°>¡±¡°<¡±»̣¡°=¡±£©0£»ÈôÆäËû̀ơ¼₫Ïàͬ£¬½ö¸Ä±äij̉»̀ơ¼₫£¬²âµĂÆäѹǿËæʱ¼äµÄ±ä»¯Èçͼ¢ñĐéÏßËùʾ£¬Ộ¸Ä±äµÄ̀ơ¼₫ÊÇ_____________£»ÔÚ5 minʱ£¬ÔÙ³äÈë0.08 mol NOºÍ0.04 molCl2£¬Ộ»́ºÏÆø̀åµÄƽ¾ùÏà¶Ô·Ö×ÓÖÊÁ¿½«_____________£῭î¡°Ôö´ó¡±¡¢¡°¼ơĐ¡¡±»̣¡°²»±ä¡±£©¡£Í¼¢̣ÊǼס¢̉̉Á½Í¬Ñ§Ăè»æÉÏÊö·´Ó¦¢ÛµÄƽºâ³£ÊưµÄ¶ÔÊưÖµ£¨lgK£©Óëζȵı仯¹Øϵͼ£¬ÆäÖĐƠưÈ·µÄÇúÏßÊÇ______£῭î¡°¼×¡±»̣¡°̉̉¡±£©£¬aֵΪ__________¡£25 ¡æʱ²âµĂ·´Ó¦¢ÛÔÚijʱ¿̀£¬NO(g)¡¢Cl2(g)¡¢NOCl(g)µÄŨ¶È·Ö±đΪ0.8¡¢0.1¡¢0.3£¬Ộ´ËʱvƠư_________vÄæ£῭î¡°>¡±¡°£¼¡±»̣¡°=¡±£©

(3)ÔÚ300 ¡æ¡¢8 MPaÏ£¬½«CO2ºÍH2°´ÎïÖʵÄÁ¿Ö®±È1¡Ă3 ͨÈë̉»ĂܱƠÈƯÆ÷ÖĐ·¢ÉúCO2(g)£«3H2(g)![]() CH3OH(g)£«H2O(g)ÖĐ·´Ó¦£¬´ïµ½Æ½ºâʱ£¬²âµĂCO2µÄƽºâת»¯ÂÊΪ50%£¬Ộ¸Ă·´Ó¦̀ơ¼₫ϵÄƽºâ³£ÊưΪKp£½_____(ÓĂƽºâ·Öѹ´ú̀æƽºâŨ¶È¼ÆËă£¬·Öѹ£½×Üѹ¡ÁÎïÖʵÄÁ¿·ÖÊư)¡£
CH3OH(g)£«H2O(g)ÖĐ·´Ó¦£¬´ïµ½Æ½ºâʱ£¬²âµĂCO2µÄƽºâת»¯ÂÊΪ50%£¬Ộ¸Ă·´Ó¦̀ơ¼₫ϵÄƽºâ³£ÊưΪKp£½_____(ÓĂƽºâ·Öѹ´ú̀æƽºâŨ¶È¼ÆËă£¬·Öѹ£½×Üѹ¡ÁÎïÖʵÄÁ¿·ÖÊư)¡£