��Ŀ����
��12�֣��ڳ����£�����������Һ����0.1mol/L NH4Cl ��0.1mol/L CH3COONH4 ��0.1mol/L NH4HSO4
�����Ҫ����д���пհף�
��1����Һ�ٳ� �ԣ���ᡱ��������С�������ԭ���ǣ�
�������ӷ���ʽ��ʾ��
��2��������������Һ�У�pH��С���� ��c(NH4+)��С���� �z����ũ{
��3�������£������Һ�ڵ�pH=7����˵��CH3COO����ˮ��̶�________���������������������NH4+��ˮ��̶ȣ�CH3COO����NH4+Ũ�ȵĴ�С��ϵ�ǣ�
c(CH3COO��) c��NH4+)���������������������
�����Ҫ����д���пհף�
��1����Һ�ٳ� �ԣ���ᡱ��������С�������ԭ���ǣ�
�������ӷ���ʽ��ʾ��
��2��������������Һ�У�pH��С���� ��c(NH4+)��С���� �z����ũ{
��3�������£������Һ�ڵ�pH=7����˵��CH3COO����ˮ��̶�________���������������������NH4+��ˮ��̶ȣ�CH3COO����NH4+Ũ�ȵĴ�С��ϵ�ǣ�
c(CH3COO��) c��NH4+)���������������������
��12�֣���ÿ��2�֣�
��1�� �� NH4++H2O NH3��H2O+H+��
NH3��H2O+H+��
��2�� �� ��
��3�� =" " =
��1�� �� NH4++H2O
 NH3��H2O+H+��
NH3��H2O+H+����2�� �� ��
��3�� =" " =
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 H����A2�����Իش��������⣺
H����A2�����Իش��������⣺ 2(OH��)
2(OH��) ��֪������CaA��ˮ�д����ܽ�ƽ�⣻CaA(s)
��֪������CaA��ˮ�д����ܽ�ƽ�⣻CaA(s) 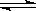 H2S +2 OH��
H2S +2 OH�� H3O+ +OH-
H3O+ +OH-  HCO3- +OH-
HCO3- +OH- ˮ��ԭ���Ʊ����ײ��ϣ���д����TiCl4�Ʊ�TiO2��xH2O�Ļ�ѧ����ʽ�� ��
ˮ��ԭ���Ʊ����ײ��ϣ���д����TiCl4�Ʊ�TiO2��xH2O�Ļ�ѧ����ʽ�� ��