ΧβΡΩΡΎ»ί
Ρ≥»ή“Κ÷–ΫωΚ§”–Na+ΓΔH+ΓΔOHΓΣΓΔCH3COO-ΥΡ÷÷άκΉ”Θ§œ¬Ν–ΥΒΖ®¥μΈσΒΡ «
cΘ®CH3COOΓΣΘ©ΘΨcΘ®Na+Θ©ΘΨcΘ®H+Θ©ΘΨcΘ®OHΓΣΘ©
| AΘ°»τ»ή“Κ÷–άκΉ”Φδ¬ζΉψΘΚcΘ®CH3COOΓΣΘ©=cΘ®Na+Θ©‘ρΗΟ»ή“Κ“ΜΕ®≥ ÷––‘ |
| BΘ°ΗΟ»ή“Κ÷–“ΜΕ®¬ζΉψΘΚ cΘ®CH3COOΓΣΘ©ΘΨcΘ®CH3COOHΘ©ΘΨcΘ®OHΓΣΘ©ΘΨcΘ®H+Θ© |
| CΘ°»ή“Κ÷–ΥΡ÷÷άκΉ”÷°ΦδΩ…Ρή¬ζΉψΘΚcΘ®Na+Θ©ΘΨcΘ®OH-Θ©ΘΨcΘ®CH3COOΓΣΘ©ΘΨcΘ®H+Θ© |
| DΘ°»τ»ή“Κ÷–ΒΡ»ή÷ «CH3COONaΚΆCH3COOHΘ§‘ρ»ή“Κ÷–άκΉ”Φδ“ΜΕ®¬ζΉψΘΚ |
BD
ΗυΨίΒγΚ… ΊΚψcΘ®Na+Θ©ΘΪcΘ®H+Θ©ΘΫcΘ®OH-Θ©ΘΪcΘ®CH3COOΓΣΘ©Ω…÷ΣΘ§A’ΐ»ΖΘ§C’ΐ»ΖΓΘBΥΒΟς»ή“Κœ‘Φν–‘Θ§“ρ¥ΥcΘ®OHΓΣΘ©”–Ω…Ρή¥σ”ΎcΘ®CH3COOHΘ©Θ§B≤Μ’ΐ»ΖΓΘ»τ»ή“Κ÷–ΒΡ»ή÷ «CH3COONaΚΆCH3COOHΘ§‘ρ»ή“Κ≤Μ“ΜΕ®œ‘Υα–‘Θ§D≤Μ’ΐ»ΖΓΘ¥πΑΗ―ΓBDΓΘ

ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
 ≈®Ε»Ήν¥σΒΡ «Θ® Θ©
≈®Ε»Ήν¥σΒΡ «Θ® Θ©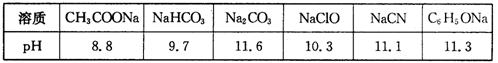



 ΓΘ”÷÷Σ≥ΘΈ¬œ¬Ρ≥CuSO4»ή“Κάο
ΓΘ”÷÷Σ≥ΘΈ¬œ¬Ρ≥CuSO4»ή“Κάο
 «ΝΕΧζΙΛ“Β÷–“ΜΗω÷Ί“ΣΖ¥”ΠΘ§ΤδΈ¬Ε»”κΤΫΚβ≥Θ ΐKΒΡΙΊœΒ»γœ¬±μΘΚ
«ΝΕΧζΙΛ“Β÷–“ΜΗω÷Ί“ΣΖ¥”ΠΘ§ΤδΈ¬Ε»”κΤΫΚβ≥Θ ΐKΒΡΙΊœΒ»γœ¬±μΘΚ
 Θ§ΜλΚœΤχΧεΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ ΓΘ
Θ§ΜλΚœΤχΧεΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ ΓΘ