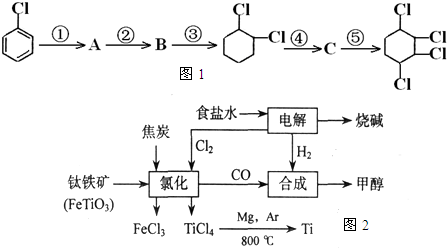
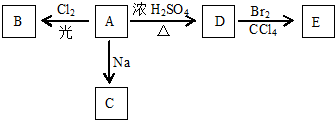
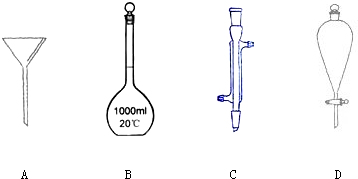
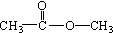��Ŀ����
��1������ʵ�������������______������ţ���
������ɫ�Լ�ƿʢ��Ũ����
����������ƽ����11.7g�Ȼ��ƾ���
����250mL����ƿ����250mL0.2mol/L��NaOH��Һ
��2��ʵ������һ��������ijͬѧ��ͨ��ʵ����������к���K+��Al3+��SO42-��3�����ӣ��밴Ҫ��ش��������⣺
��ͬѧ��������������ˮ�Ƴ���Һ��������Һ�ֳ����ݣ����õ�1����Һ������K+�IJ����������ýྻ�IJ�˿պȡ������Һ���ھƾ��ƻ��������գ�����ɫ�ܲ����۲�����ɫΪ______ɫ��
���õ�2����Һ������Al3+��������ѡ�õķ�Ӧ�Լ�Ϊ______��
�ڼ���SO42-ʱ����ͬѧ���õ�ʵ�鲽���ǣ������3����Һ�м��������ϡ���Ტ��ֻ�ϣ��ٵ���BaCl2��Һ�����ȼ���ϡ�����Ŀ����______��
������ɫ�Լ�ƿʢ��Ũ����
����������ƽ����11.7g�Ȼ��ƾ���
����250mL����ƿ����250mL0.2mol/L��NaOH��Һ
��2��ʵ������һ��������ijͬѧ��ͨ��ʵ����������к���K+��Al3+��SO42-��3�����ӣ��밴Ҫ��ش��������⣺
��ͬѧ��������������ˮ�Ƴ���Һ��������Һ�ֳ����ݣ����õ�1����Һ������K+�IJ����������ýྻ�IJ�˿պȡ������Һ���ھƾ��ƻ��������գ�����ɫ�ܲ����۲�����ɫΪ______ɫ��
���õ�2����Һ������Al3+��������ѡ�õķ�Ӧ�Լ�Ϊ______��
�ڼ���SO42-ʱ����ͬѧ���õ�ʵ�鲽���ǣ������3����Һ�м��������ϡ���Ტ��ֻ�ϣ��ٵ���BaCl2��Һ�����ȼ���ϡ�����Ŀ����______��
��1����Ũ��������ֽ⣬Ӧ�ô������ɫ�Լ�ƿ�У��ʢٴ���
������������ƽ����ȷ��0.1g���ʿ�����������ƽ����11.7g�Ȼ��ƾ��壬�ʢ���ȷ��
������250mL 0.2mol/L��NaOH��Һ������ѡ����250mL����ƿ���ʢ���ȷ��
�ʴ�Ϊ���ڢۣ�
��2����ɫ��Ӧ�������ӳ�����ɫ���棻��������������������ǿ����Һ��ѡ���������Ƽ��������ӣ����������ʹ������ϡ���ᣬ�ų�Ag+��CO32-��SO32-�����ӵĸ��ţ�
�ʴ�Ϊ���ϣ�NaOH��Һ���ų�Ag+��CO32-��SO32-�����ӵĸ��ţ�
������������ƽ����ȷ��0.1g���ʿ�����������ƽ����11.7g�Ȼ��ƾ��壬�ʢ���ȷ��
������250mL 0.2mol/L��NaOH��Һ������ѡ����250mL����ƿ���ʢ���ȷ��
�ʴ�Ϊ���ڢۣ�
��2����ɫ��Ӧ�������ӳ�����ɫ���棻��������������������ǿ����Һ��ѡ���������Ƽ��������ӣ����������ʹ������ϡ���ᣬ�ų�Ag+��CO32-��SO32-�����ӵĸ��ţ�
�ʴ�Ϊ���ϣ�NaOH��Һ���ų�Ag+��CO32-��SO32-�����ӵĸ��ţ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
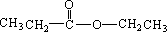 ����
����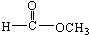 ��
��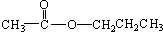 ����
����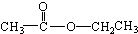 ��
��