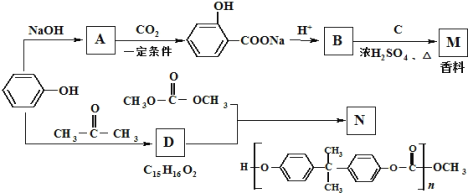
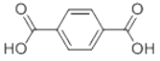
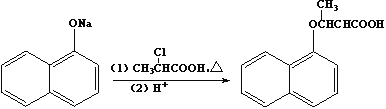
��Ŀ����
����Ŀ���������б��ش��й����⣺
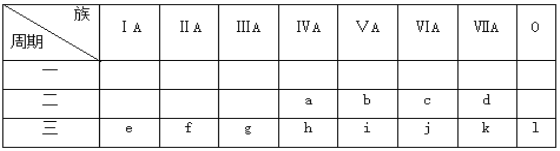
��1���ϱ��У���һ��������С��Ԫ����__(��Ԫ�����ƣ���ͬ)���縺������Ԫ����__��
��2��ijԪ������������Ӧ��ˮ��������ԣ�д����Ԫ����cԪ���γɵĻ�������NaOH��Һ��Ӧ�����ӷ���ʽ__��
��3����e��kԪ�ص�ԭ���У�ԭ�Ӱ뾶��С����___(��Ԫ�ط���)����۵����Ų�ʽΪ__��δ�ɶԵ�����������__(��Ԫ�ط���)��������������Ӧˮ����Ļ�ѧʽΪ__��������δ�ɶԵ��ӵ�Ԫ����__(��Ԫ�ط���)��M���������չ������__(��Ԫ�ط���)��
���𰸡��� �� Al2O3+2OH-=2AlO2-+H2O Cl 3s23p5 P H3PO4 Si��S Al
��������
����Ԫ�������ڱ��е�λ�ÿ�֪��aΪCԪ�أ�bΪNԪ�أ�cΪOԪ�أ�dΪFԪ�أ�eΪNaԪ�أ�fΪMgԪ�أ�gΪA1Ԫ�أ�hΪSiԪ�أ�iΪPԪ�أ�jΪSԪ�أ�kΪC1Ԫ�أ�lΪArԪ�أ����Ԫ�������ɷ������
��1��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�������A�塢��VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ�ͬһ����Ԫ���У����һ����������ԭ���������������С����һ��������С��Ԫ�����ƣ�ͬ����������ҵ縺������ͬ�������϶��µ縺�Խ��ͣ��縺������Ԫ���Ƿ����ʴ�Ϊ���ƣ�����
��2��ijԪ������������Ӧ��ˮ��������ԣ��û�����Ϊ�������������Ԫ����g����Ԫ����cԪ���γɵĻ�����Ϊ�������������������������NaOH��Һ��Ӧ����Ӧ�����ӷ���ʽΪ��![]() ��
��
��3��e��kԪ������ͬ����Ԫ�أ�ͬһ���ڣ��������ң�ԭ�Ӱ뾶��С��ԭ�Ӱ뾶��С����Cl��Ϊ17��Ԫ�أ���۵����Ų�ʽΪ3s23p5��δ�ɶԵ��������ļ۵����Ų�ʽΪ3s23p3����PԪ�أ�������������Ӧˮ����Ļ�ѧʽΪH3PO4��������δ�ɶԵ��ӵ�Ԫ�صļ۵����Ų�ʽΪ3s23p2��3s23p4��ΪSi��SԪ�أ�M���������չ���ļ۵����Ų�ʽΪ3s23p1����AlԪ�ء�

����Ŀ����Ҫ�����
���� | �ṹ��ʽ | ||
��1�� | ___ | ��6�������� | ___ |
��2�� | ___ | ��7��������������ܶ�Ϊ46��ij������ | ___ |
��3�� | ___ | ��8������ | ___ |
��4�� | ___ | ��9������Ҫ�Ļ���ʯ��ˮƽ������ | ___ |
��5��HCHO | ___ | ��10��̼ԭ������3-6����������һ�ȴ���ֻ��һ�� | ___ |
����Ŀ��ij��θҩ��ֹ���Ϊ̼��ƣ��ⶨÿƬ��̼��ƺ����ķ��������¼�����������ҩƬ�е������ɷֲ������ᷴӦ��Ҳ�����������Ʒ�Ӧ����ʵ�鲽�����£�
������![]() ϡ�����
ϡ�����![]() ��Һ��
��Һ��![]()
��ȡһ��ҩƬ��![]() ����������
����������![]() ����ˮ
����ˮ
�ۼ���![]() ϡ����
ϡ����
����![]() ��Һ�к������ᣬ��ȥ���Ϊ
��Һ�к������ᣬ��ȥ���Ϊ![]() ��
��
��ش��������⣺
��1���ⶨ�����з�����Ӧ�����ӷ���ʽ_________��
��2����������![]() ϡ�������ò�����������Ͳ���ձ���________��
ϡ�������ò�����������Ͳ���ձ���________��
��3�����ѡ�÷�̪��ָʾ�����ζ��ﵽ�յ������Ϊ____��
��4��ijͬѧ�Ĵβⶨ��![]() �������£�
�������£�
�ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| 13.40 | 11.90 | 12.10 | 12.00 |
�������λͬѧ��ʵ�����ݣ�����ҩƬ��̼��Ƶ���������Ϊ_____��
��5������ʵ������д������в�������ʹ����̼��Ƶ���������ƫ�ߵ���_________��
a ��û����ϴ�ļ�ʽ�ζ���װ![]() ��Һ���еζ�
��Һ���еζ�
b ��û����ϴ����ʽ�ζ�����ȡ![]() ϡ�����ܽ���Ʒ
ϡ�����ܽ���Ʒ
c ��![]() ��Һ�ζ�ʱ����ʼ����ƽ�ӣ��յ㸩��
��Һ�ζ�ʱ����ʼ����ƽ�ӣ��յ㸩��
d װ![]() ��Һ�ĵζ��ܣ��ζ�ǰ���������ݣ��ζ������������ݡ�
��Һ�ĵζ��ܣ��ζ�ǰ���������ݣ��ζ������������ݡ�