��Ŀ����
Ϊ�ⶨ����Na2O���ʵ�Na2O2��Ʒ�Ĵ��ȣ��ס��Ҷ�λͬѧ����˶��ֲ�ͬ��ʵ�鷽������֪��2Na2O2+2CO2�T2Na2CO3+O2
2Na2O2+2H2O�T4NaOH+O2
�ף���ͼl��ʾװ�ã�ͨ���ⶨNa2O2��CO2��Ӧ����O2��������ⶨ��Ʒ�Ĵ��ȣ�
��1��C����ʢ��ҩƷ�ǣ�______��
��2��A����Ƥ�ܵ������ǣ�______��
��3�����ø�ʵ�鷽������Na2O2�Ĵ�������ƫ����ԭ�������______����ѡ����ĸ����
a��װ��A��B�еĿ����Բⶨ���������Ӱ��
b��װ��C�еĿ����Բⶨ���������Ӱ��
c������ʱU���������е�Һ������ҵ�
d������ʱU���������е�Һ������Ҹ�
�ң���ȡ3.500g���������1000.00mL��Һ����0.1000mol?L-1�ı�����ζ���
��4��ȡ����������Һ25.00mL����ƿ�У���������ͼ��ʾ���ֲֳ���ʡ�ԣ�����ȷ�IJ�����ͼ______��ȡ��Һ����������������______��

��5���ζ�����ƽ��ʵ������ݼ�¼���±���
| �ζ����� | ��һ�εζ� | �ڶ��εζ� | �����εζ� |
| ���ı�����������mL�� | 24.98 | 25.00 | 25.02 |
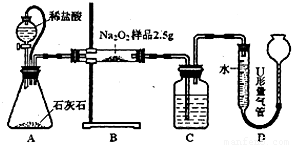
���𰸡���������1�����������ܺͶ�����̼��Ӧ������������Ӧ��
��2����Ƥ�ܿ��Թ�ͨ��ƿ�ͷ�Һ©���еĴ���ѹǿ�������Ա��������ſ�����
��3�������������������ʵ��ֵ�ߣ���������ƵĴ���װ�þͻ�ƫ�ߣ�
��4����������ƿ��ȡҺ�Ļ���ʵ������жϣ�ȡҺ�õ�����Һ�ܣ�
��5�����ݱ������ı�Һ������ݣ��������Һ��ƽ����������ù�ϵʽ��Na2O2��2HCl��Na2O��2HCl������������ơ��������ܵ����ʵ����������������ݣ���ʽ����������ơ��������Ƹ��Ե����ʵ��������������������Ƶ�����������
����⣺��1��C�����������ն�����̼����֤��ˮ��������õ��������������
�ʴ�Ϊ��NaOH��Һ��
��2��A����Ƥ�ܿ��Թ�ͨ��ƿ�ͷ�Һ©�������ѹ���ã�ʹ���������£����ҿ��Ա��������ſ���ʹ�������ƫ��
�ʴ�Ϊ�����ѹ���ã�ʹ���������£����������ſ���ʹ�������ƫ��
��3��a��װ��A��B�еĿ����ᵼ�²�õ��������ƫ�ߣ��Բⶨ�������Ӱ�죬��a��ȷ��
b���ڵ���U���������е�Һ����ƽ�Ĺ����У��ų���װ��C�еĿ����Բⶨ���������Ӱ�죬��b����
c������ʱU���������е�Һ��Ҫ������ƽ������ҵͣ�����Na2O2�Ĵ��Ȼ�ƫ��c��ȷ��
d������ʱU���������е�Һ������Ҹ�ʱ������Na2O2�Ĵ��Ȼ�ƫС����d����
��ѡac��
��4��ͼ�����õ���������Һ�ܣ�����Һ�ܷ��������Һ�������У�ʹ���ڼ�˿��������ڱڣ���������б����Һ���ִ�ֱ���ſ�ʳָ��ʹ��Һ�������ڱ���Ȼ���£�����Һ������Һ�������ٵȴ�15�룬ȡ����Һ�ܣ����ڹܿڵ�����Һ�岻Ҫ������
�ʴ��ǣ�D����Һ�ܣ�
��5�����εζ����ı�Һ���������Ч�ģ��������ĵı�Һ�����ƽ������ǣ�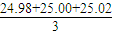 mL=25mL���������ơ������ƺ����ᷴӦ�Ĺ�ϵʽ�ǣ�Na2O2��2HCl��Na2O��2HCl���������ƺ��������ܵ����ʵ����ǣ�n��Na2O2��=
mL=25mL���������ơ������ƺ����ᷴӦ�Ĺ�ϵʽ�ǣ�Na2O2��2HCl��Na2O��2HCl���������ƺ��������ܵ����ʵ����ǣ�n��Na2O2��= n��HCl��=0.5×0.025L×0.1000mol?L-1=0.00125mol��1000.00mL��Һ��Һ�к��е������ƺ��������ܵ����ʵ����ǣ�0.00125×
n��HCl��=0.5×0.025L×0.1000mol?L-1=0.00125mol��1000.00mL��Һ��Һ�к��е������ƺ��������ܵ����ʵ����ǣ�0.00125×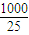 =0.05mol��
=0.05mol��
���������xmol�������������ʵ����ǣ�0.05-x��mol��
����������ϵ�ɵã�78x+62×��0.05-x��=3.500��
���x=0.025mol���������Ƶ����������ǣ� ×100%=55.7%��
×100%=55.7%��
�ʴ��ǣ�55.7%��
���������⿼��ѧ��ʵ�������Լ��������Ƶ����ʣ�Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�
��2����Ƥ�ܿ��Թ�ͨ��ƿ�ͷ�Һ©���еĴ���ѹǿ�������Ա��������ſ�����
��3�������������������ʵ��ֵ�ߣ���������ƵĴ���װ�þͻ�ƫ�ߣ�
��4����������ƿ��ȡҺ�Ļ���ʵ������жϣ�ȡҺ�õ�����Һ�ܣ�
��5�����ݱ������ı�Һ������ݣ��������Һ��ƽ����������ù�ϵʽ��Na2O2��2HCl��Na2O��2HCl������������ơ��������ܵ����ʵ����������������ݣ���ʽ����������ơ��������Ƹ��Ե����ʵ��������������������Ƶ�����������
����⣺��1��C�����������ն�����̼����֤��ˮ��������õ��������������
�ʴ�Ϊ��NaOH��Һ��
��2��A����Ƥ�ܿ��Թ�ͨ��ƿ�ͷ�Һ©�������ѹ���ã�ʹ���������£����ҿ��Ա��������ſ���ʹ�������ƫ��
�ʴ�Ϊ�����ѹ���ã�ʹ���������£����������ſ���ʹ�������ƫ��
��3��a��װ��A��B�еĿ����ᵼ�²�õ��������ƫ�ߣ��Բⶨ�������Ӱ�죬��a��ȷ��
b���ڵ���U���������е�Һ����ƽ�Ĺ����У��ų���װ��C�еĿ����Բⶨ���������Ӱ�죬��b����
c������ʱU���������е�Һ��Ҫ������ƽ������ҵͣ�����Na2O2�Ĵ��Ȼ�ƫ��c��ȷ��
d������ʱU���������е�Һ������Ҹ�ʱ������Na2O2�Ĵ��Ȼ�ƫС����d����
��ѡac��
��4��ͼ�����õ���������Һ�ܣ�����Һ�ܷ��������Һ�������У�ʹ���ڼ�˿��������ڱڣ���������б����Һ���ִ�ֱ���ſ�ʳָ��ʹ��Һ�������ڱ���Ȼ���£�����Һ������Һ�������ٵȴ�15�룬ȡ����Һ�ܣ����ڹܿڵ�����Һ�岻Ҫ������
�ʴ��ǣ�D����Һ�ܣ�
��5�����εζ����ı�Һ���������Ч�ģ��������ĵı�Һ�����ƽ������ǣ�
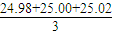 mL=25mL���������ơ������ƺ����ᷴӦ�Ĺ�ϵʽ�ǣ�Na2O2��2HCl��Na2O��2HCl���������ƺ��������ܵ����ʵ����ǣ�n��Na2O2��=
mL=25mL���������ơ������ƺ����ᷴӦ�Ĺ�ϵʽ�ǣ�Na2O2��2HCl��Na2O��2HCl���������ƺ��������ܵ����ʵ����ǣ�n��Na2O2��= n��HCl��=0.5×0.025L×0.1000mol?L-1=0.00125mol��1000.00mL��Һ��Һ�к��е������ƺ��������ܵ����ʵ����ǣ�0.00125×
n��HCl��=0.5×0.025L×0.1000mol?L-1=0.00125mol��1000.00mL��Һ��Һ�к��е������ƺ��������ܵ����ʵ����ǣ�0.00125×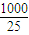 =0.05mol��
=0.05mol�����������xmol�������������ʵ����ǣ�0.05-x��mol��
����������ϵ�ɵã�78x+62×��0.05-x��=3.500��
���x=0.025mol���������Ƶ����������ǣ�
 ×100%=55.7%��
×100%=55.7%���ʴ��ǣ�55.7%��
���������⿼��ѧ��ʵ�������Լ��������Ƶ����ʣ�Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ���أ�
���أ� ������ɰ��Na2B4O7���ڸ��¸�ѹ�·�Ӧ���Ի�������
������ɰ��Na2B4O7���ڸ��¸�ѹ�·�Ӧ���Ի�������