��Ŀ����
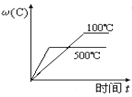
����Ŀ����ҵ���Ʊ��ϳ����Ĺ�����Ҫ��ˮ�����������飺 CH4(g)��H2O(g)CO(g)��3H2(g) ��H��0����һ�������£������Ϊ1L���ܱ������г���1 molCH4(g)��1molH2O(g)����� H2O(g)�� H2(g)��Ũ����ʱ��仯������ͼ��ʾ������˵����ȷ���� (����)
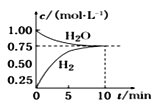
A.�ﵽƽ��ʱ��CH4(g)��ת����Ϊ75%
B.0��10 min�ڣ�v(CO)��0.075 mol��L-1��min -1
C.�÷�Ӧ�Ļ�ѧƽ�ⳣ��K��0.1875
D.��CH4(g)������������H2(g)�������������ʱ����Ӧ����ƽ��
���𰸡�C
��������
��ͼ��֪��10minʱ��Ӧ����ƽ�⣬ƽ��ʱˮ��������Ũ�Ⱦ�Ϊ0.75mol/L����

A��ƽ��ʱ����ת����=![]() ��100%=25%����A����
��100%=25%����A����
B��010min�ڣ�v(CO)= ![]() ��0.025molL1min1����B����
��0.025molL1min1����B����
C��ƽ�ⳣ��K= =0.1875��
=0.1875��
��C��ȷ��
D��ͬһ���ʵ������������������������ʱ����Ӧ����ƽ�⣬�ɷ���ʽ��֪��CH4(g)������������H2(g)����������Ϊ1:3ʱ����Ӧ����ƽ�⣬��D����
��ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������β���к���CO��NOx���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת��Ϊ�����塣
��1����֪ 4CO(g)��2NO2(g)![]() 4CO2(g)��N2(g) ��H= -1200kJ��mol-1
4CO2(g)��N2(g) ��H= -1200kJ��mol-1
�ٸ÷�Ӧ��________________������¡����»��κ��¶ȡ��������Է����С�
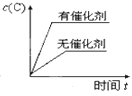
�ڶ��ڸ÷�Ӧ���ı�ijһ��Ӧ�������¶�T1>T2��������ͼ����ȷ����_______(�����)��

��ijʵ��С��ģ�������������̣�һ���¶��£��� 2L�ĺ����ܱ������У���ʼʱ���ռס������ַ�ʽ����Ͷ�ϣ�����һ��ʱ���ﵽƽ��״̬����ü���CO��ת����Ϊ50%����÷�Ӧ��ƽ�ⳣ��Ϊ__________�����ַ�ʽ��ƽ��ʱ��N2�������������______�ң� �>��=��<��ȷ��������ͬ����NO2��Ũ�ȣ���______�ҡ�
�� | �� |
0.2mol NO2 | 0.1mol NO2 |
0.4mol CO | 0.2mol CO |
��2����������β���е�̼��(C)��NOx��ͨ��ij���ܴ�������������ͬ�¶��£���ģ��β�����ɷ����±���ʾ������ͬ������ͨ���ô���������в���(CO2��N2��N2O)��NO��������ݽ����ͼ��ʾ��
ģ��β�� | ���壨10mol�� | ̼�� | ||
NO | O2 | He | ||
���ʵ�����mol�� | 0.025 | 0.5 | 9.475 | a |

��375��ʱ������ų��������к�0.45mol O2��0.0525mol CO2����Y�Ļ�ѧʽΪ________��
��ʵ������в���NOģ��NOx����������NO2��ԭ����______________________��