��Ŀ����
�ҹ���ѧ�Һ�°�ĸ����Ĵ�����������,�������̿ɼ�Ҫ��ʾ��ͼ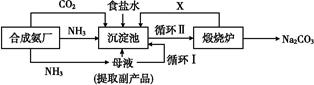
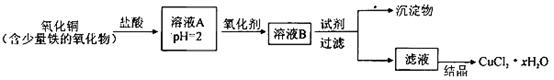
(1)������������ķ�������������,����Ʒ��һ����;Ϊ����������
(2)�������з����Ļ�ѧ��Ӧ����ʽ�� ����
(3)д������������X���ʵķ���ʽ�� ��
(4)ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%����,��Ҫ���������������(�����������еı��)��ѭ�����ӳ�������ȡ�������IJ������������� ��
(5)Ϊ�����Ʒ̼�������Ƿ����Ȼ���,��ȡ������������ˮ��,�ٵμ�����������
(6)��ĸҺ��ͨ����,����ϸСʳ�ο���,��ȴ��������Ʒ,ͨ����������������������
a.����NH4+��Ũ��,ʹNH4Cl���������
b.ʹNaHCO3���������
c.ʹNaHCO3ת��ΪNa2CO3,���������NH4Cl����
(1)�����Ƽ���°��Ƽ���������ʻ���Һ��ҩ��(���������𰸾�����)
(2)NH3+CO2+H2O+NaCl NH4Cl+NaHCO3��
NH4Cl+NaHCO3��
(3)CO2��(4)I������
(5)ϡ�������������Һ��(6)ac
����

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д����ȷ�Ӧ������ұ�����۵�Ľ������ɼ���Ϊ������ijЩ�����������ڸ��������·����ķ�Ӧ��ijѧϰС������ȷ�Ӧ����Al��Fe2O3��ӦΪ����ʵ������о���
�������ݵõ�Al��Al2O3��Fe��Fe2O3���۵㡢�е��������±���ʾ��
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1565[ |
| �е�/�� | 2467 | 2980 | 2750 | �� |
�Իش��������⣺
��1�������ȷ�Ӧ�н��������ֳ� �ԣ����������ԭ���������ж����н�������һ�����������ȷ�Ӧ��ȡ ��������ţ�
��Fe����Cr����������V����������Ca����Mn
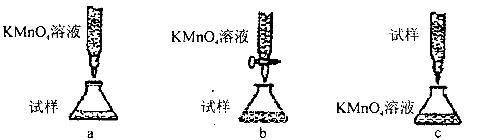
��2��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ�����������Ӧ�������������ֽ����������һ����ʵ�鷽��֤�����������к��н���������ʵ�������õ��Լ�Ϊ ���ɹ۲쵽��ʵ�������� ��
��3����һͬѧ�Ʋ����ȷ�Ӧ�õ����������л�����Fe2O3������������·�������֤��
ȡһ���������Ͷ�뵽����ϡ�����У���Ӧ��Ļ��Һ�еμ����ʼ���Һ���۲���Һ��ɫδ��Ѫ��ɫ������֤���������в�����Fe2O3����
�����ʼ��� ���ѧʽ����
�ڸ�ͬѧ��ʵ�鷽���Ƿ������ ���������������������
���ɣ�

 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��
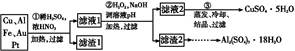
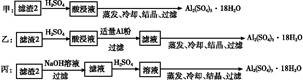
 CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;
CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;  xH2O)�������²�����
xH2O)�������²�����