��Ŀ����
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2��SO4��3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�
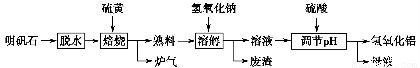
��1���������������з�Ӧ�Ļ�ѧ����ʽΪ Al2��SO4��3��
Al2��SO4��3�� S
S
 Al2O3��
Al2O3�� ______����
______����
��2��������Һ�ͷ����IJ�����________�����ܽ���ʱ��Ӧ�����ӷ���ʽΪ_________________________________________________________________��
��3��������pH������ˡ�ϴ��Al��OH��3������֤��������ϴ�Ӹɾ���ʵ�������������________��
��4����ĸҺ���пɻ��յ�������________��
��5������������������ǡ������48 g���ƣ������������տɵõ�________ g����������
��1��2��3��2��9��SO2
��2�����ˡ�Al2O3��2OH��=2AlO3-��H2O
��3��ȡ���һ��ϴ�ӵ���Һ���Թ������μ�BaCl2��Һ�����ް�ɫ������������֤����ϴ�Ӹɾ�
��4��K2SO4��Na2SO4
��5��468
����������1��������֪�ķ�Ӧ����������֪��ֻ����Ԫ�ػ��ϼ۷����仯��������ֻ��������SO2����2��������̿�֪��������Һ�ͷ������ù��˲������ܽ�ʱ��Al2O3��Ӧ����3��Al��OH��3��������������SO42-���ʿ�ȡ���һ��ϴ�ӵ���Һ���Թ������μ�BaCl2��Һ�����ް�ɫ����������֤����ϴ�Ӹɾ�����4����ĸҺ���пɻ��յ����������ܵ�K2SO4��Na2SO4����5�����ݷ�Ӧ�Ļ�ѧ����ʽ��֪��2Al2��SO4��3��3S��n��S����1.5 mol��n[Al2��SO4��3]��1 mol��n��Al2O3����1 mol������ʯ��n��Al2O3����2 mol��������Ԫ���غ��֪��n��Al����n[Al��OH��3]��6 mol����������������Ϊ468 g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�