��Ŀ����
��6�֣���ͼ���³İ�ֽ�IJ���Ƭ�������������KmnO4����������Χ�ֱ�μ�һ��KBr��Һ��Ʒ����Һ�����з�̪�ij���ʯ ��ˮ��FeC12��Һ��Ȼ����KMnO4�����ϵμ�������Ũ���ᣬѸ�ٸǺñ�����
��ˮ��FeC12��Һ��Ȼ����KMnO4�����ϵμ�������Ũ���ᣬѸ�ٸǺñ�����
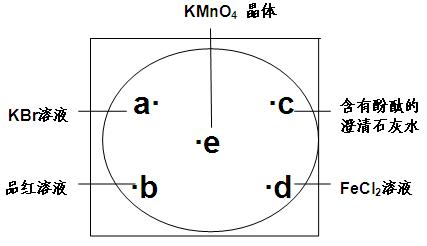
��ʾ��ʵ���������õ�������������������ԭ����ȡ��
2KMnO4+16HC1��Ũ�� 2KC1+2MnC12+5C12��+8H2O ���˷�Ӧ�ڳ����¼��ܽ��С�
��1��a����Ӧ���ӷ���ʽ�� ��
��2��b���������� ��d���������� ��
��3��c����Ӧ�Ļ�ѧ��Ӧ�ڹ�ҵ���ô�ԭ����ȡƯ�ۣ�����ȡ��������ʯ��������ñ�
��ʯ��ˮ��ԭ���� ��
 ��ˮ��FeC12��Һ��Ȼ����KMnO4�����ϵμ�������Ũ���ᣬѸ�ٸǺñ�����
��ˮ��FeC12��Һ��Ȼ����KMnO4�����ϵμ�������Ũ���ᣬѸ�ٸǺñ�����
��ʾ��ʵ���������õ�������������������ԭ����ȡ��
2KMnO4+16HC1��Ũ��
��1��a����Ӧ���ӷ���ʽ�� ��
��2��b���������� ��d���������� ��
��3��c����Ӧ�Ļ�ѧ��Ӧ�ڹ�ҵ���ô�ԭ����ȡƯ�ۣ�����ȡ��������ʯ��������ñ�
��ʯ��ˮ��ԭ���� ��
��6�֣�
��1��C12+2Br-=2C1-+Br2��2�֣�
��2����ɫ��dz������ȥ����dz��ɫ��Ϊ��ɫ����1�֣���2�֣�
��3������ʯ��ˮŨ��̫С�� ����̫�������ڹ�ҵ������2�֣�
����̫�������ڹ�ҵ������2�֣�
��1��C12+2Br-=2C1-+Br2��2�֣�
��2����ɫ��dz������ȥ����dz��ɫ��Ϊ��ɫ����1�֣���2�֣�
��3������ʯ��ˮŨ��̫С��
 ����̫�������ڹ�ҵ������2�֣�
����̫�������ڹ�ҵ������2�֣���

��ϰ��ϵ�д�
�����Ŀ
 MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��