��Ŀ����
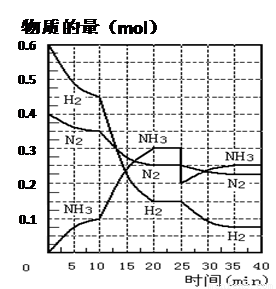
 ���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4KJ?mol-1������500�桢20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ���ش��������⣺
���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4KJ?mol-1������500�桢20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ���ش��������⣺��1��10min����NH3��ʾ��ƽ����Ӧ����
��2����10��20min�ڣ�NH3Ũ�ȱ仯��ԭ�������
A�����˴��� B����С������� C�������¶� D������NH3���ʵ���
��3����1��ƽ���ʱ�䷶ΧΪ��
| c(NH3)2 |
| c(N2)��c(H2)3 |
| c(NH3)2 |
| c(N2)��c(H2)3 |
��4���ڷ�Ӧ������25minʱ�����߷����仯��ԭ��
| ||
| ��t |
��2������ͼ��֪��ƽ��������Ӧ�����ƶ���10minʱ�������ģ������������ʵ��������ӱ�����ͬ��˵��Ϊʹ�ô�����
��3���ﵽƽ��״̬ʱ�����ʵ������䣬�Դ��жϴﵽƽ���ʱ��Σ���ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���2��ƽ��ʱNH3������������ڰ����ĺ�����
��4��25���ӣ�NH3�����ʵ���ͻȻ���٣���H2��N2�����ʵ������䣬˵��Ӧ�Ƿ����NH3��
| ||
| ��t |
| ||
| 10min |
�ʴ�Ϊ��0.005mol/��L��min����
��2����ͼ��֪��0-10�����ڡ�n��N2��=0.025mol��2=0.05mol��10-20�����ڡ�n�䣨N2��=0.025mol��4=0.1mol������֮�ȵ������ʵ���֮�ȣ����ԣ�0-10������10��20��������ʱ����У�N2�ķ�Ӧ����֮��Ϊ0.05mol��0.1mol=1��2��
��ͼ���֪��������ʵ����仯���죬��10minʱ�仯�������ģ�20min��ƽ��ʱ����n�䣨N2��=0.025mol��4=0.1mol��
��n��H2��=0.025mol��12=0.3mol����n��NH3��=0.025mol��8=0.2mol�����ʵ����仯֮�ȵ��ڻ�ѧ������֮�ȣ������������ʵ��������ӱ�����ͬ��˵��10min���ܸı��������ʹ�ô�����
��ѡA��
��3����ͼ����Կ���������Ӧ���е�ʱ20-25min�������ʵ������䣬˵����Ӧ�ﵽƽ��״̬��
��ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ�������ƽ�ⳣ��K=
| c(NH3)2 |
| c(N2)��c(H2)3 |
��2��ƽ��ʱNH3���������=
| 2.5mol |
| 2.5mol+2.25mol+0.75mol |
�ʴ�Ϊ��20-25min��
| c(NH3)2 |
| c(N2)��c(H2)3 |
��4����25���ӣ�NH3�����ʵ���ͻȻ���٣���H2��N2�����ʵ������䣬˵��Ӧ�Ƿ����NH3��
�ʴ�Ϊ�������0.1molNH3��

 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
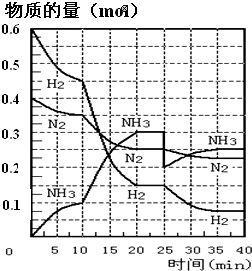
��Ԫ����ĩ��ϰ�ȷ��ϵ�д���27�֣����ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��![]() 2NH3��g�� ����H=��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺
2NH3��g�� ����H=��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺

��1��10min����NH3��ʾ��ƽ����Ӧ���� ��
��2����10~20 min�ڣ�NH3Ũ�ȱ仯��ԭ������� ��
A�����˴��� B����С�������
C�������¶� D������NH3���ʵ���
��3����1��ƽ���ʱ�䷶ΧΪ�� ��
��2��ƽ���ʱ�䷶ΧΪ�� ��
��1��ƽ�⣺ƽ�ⳣ��K1 = �������ݵı���ʽ����
��2��ƽ��ʱNH3��������� ��
��4���ڷ�Ӧ������25 minʱ��
�� ���߷����仯��ԭ��
�� ��ڶ���ƽ��ʱ����ƽ���ƽ�ⳣ��
K2 K1������ڡ��������ڡ�����С�ڡ�����
��5����25~40minʱ������ϳɰ������еķ�Ӧ�ȡ�H= ��
��6���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ�������з�Ӧ��
N2��g��+ 3H2O��1�� ![]() 2NH3��g��+
2NH3��g��+ ![]() O2��g�� ����H = a kJ��mol��1
O2��g�� ����H = a kJ��mol��1
��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| T/K | 303 | 313 | 323 |
| NH3������/��10-6mol�� | 4��8 | 5��9 | 6��0 |
�ٴ˺ϳɷ�Ӧ��a 0����S 0�����������������������
����֪��N2��g��+ 3H2��g��![]() 2NH3��g�� ��H= ��92 ��4kJ��mol��1
2NH3��g�� ��H= ��92 ��4kJ��mol��1
2H2��g��+ O2��g�� = 2H2O��l��= ��571��6kJ��mol��1
�����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ��
��17�֣����ĺϳ�ԭ��Ϊ��N2��g��+3H2��g�� 2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ��
2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ��
�ش��������⣺
��1��10 min����NH3��ʾ��ƽ����Ӧ���� ��
��2����10 ~20 min�ڣ�NH3Ũ�ȱ仯��ԭ������� ��
| A�����˴��� | B����С������� | C�������¶� | D������NH3���ʵ��� |
��1��ƽ�⣺ƽ�ⳣ��K1 = �������ݵı���ʽ������2��ƽ��ʱNH3��������� ��
��4���ڷ�Ӧ������25 minʱ��
�����߷����仯��ԭ��
�ڴ�ڶ���ƽ��ʱ����ƽ���ƽ�ⳣ��K2 K1������ڡ��������ڡ�����С�ڡ�����
��5����25~40 minʱ������ϳɰ������еķ�Ӧ�ȡ�H= ��
��6���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ�������з�Ӧ��
N2��g�� + 3H2O��1��
 2NH3��g�� +
2NH3��g�� +  O2��g�� ����H =" a" kJ��mol��1
O2��g�� ����H =" a" kJ��mol��1��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| T/K | 303 | 313 | 323 |
| NH3������/��10-6mol�� | 4��8 | 5��9 | 6��0 |
����֪��N2��g�� + 3H2��g��
 2NH3��g�� ��H= ��92 ��4kJ��mol��1
2NH3��g�� ��H= ��92 ��4kJ��mol��12H2��g�� + O2��g�� = 2H2O��l�� = ��571��6kJ��mol��1
�����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ��
���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g�� 2NH3��g�� ����H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺
2NH3��g�� ����H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺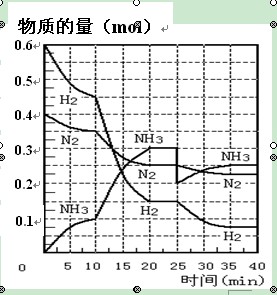
��1��10 min����NH3��ʾ��ƽ����Ӧ���� ��
��2����10 ~20 min�ڣ�NH3Ũ�ȱ仯��ԭ������� ��
| A�����˴��� | B����С������� | C�������¶� | D������NH3���ʵ��� |
��4���ڷ�Ӧ������25 minʱ�� ���߷����仯��ԭ��
 2NH3(g)
����H= ��92.4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺
2NH3(g)
����H= ��92.4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺