��Ŀ����
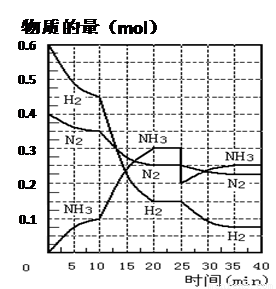
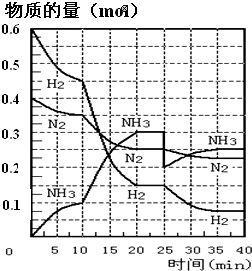
���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g�� 2NH3��g�� ����H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺
2NH3��g�� ����H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺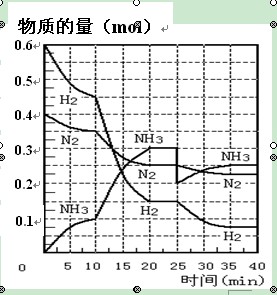
��1��10 min����NH3��ʾ��ƽ����Ӧ���� ��
��2����10 ~20 min�ڣ�NH3Ũ�ȱ仯��ԭ������� ��
| A�����˴��� | B����С������� | C�������¶� | D������NH3���ʵ��� |
��4���ڷ�Ӧ������25 minʱ�� ���߷����仯��ԭ��
��12�֣�ÿ��2�֣���1�� v = 0��005 mol��L-1 ��min-1 ��û�е�λ�����֣�
��2�� AB ��©ѡ��1�֣���ѡ�����֣�
��3���� 20~25 min K1 = 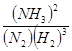 45��5 %
45��5 %
��4�� ����0��1 mol NH3��û��ָ��0.1mol�ĸ�1�֣�
���������������1������ͼ���֪��10minʱ���������ʵ�����0.1mol���������ķ�Ӧ������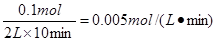 ��
��
��2������ͼ���֪����10 ��20 min��NH3Ũ�����ӣ�������������Ũ�ȼ�С����˵����Ӧ������Ӧ������С���Ϊ�÷�Ӧ�������С�ġ����ȵĿ��淴Ӧ�����Ըı��������������С����������¶ȣ���ѡBC��
��3������ͼ���֪����20 ��25 min�ڸ������ʵ�Ũ�Ȳ��ٷ����仯������Ӧ�ﵽƽ��״̬�����Ե�1��ƽ���ʱ�䷶ΧΪ20 ��25 min����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ�����Ը��ݷ�Ӧ�ķ���ʽ��֪���÷�Ӧ��ƽ�ⳣ��K1��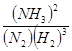 ��ͬ������ͼ���֪����Ӧ���е�35minʱ���������ʵ�Ũ�Ȳ��ٷ����仯������Ӧ�ﵽƽ��״̬����ʱ�����������Ͱ��������ʵ����ֱ���0.075mol��0.225mol��0.25mol�����Ե�2��ƽ��ʱNH3�����������
��ͬ������ͼ���֪����Ӧ���е�35minʱ���������ʵ�Ũ�Ȳ��ٷ����仯������Ӧ�ﵽƽ��״̬����ʱ�����������Ͱ��������ʵ����ֱ���0.075mol��0.225mol��0.25mol�����Ե�2��ƽ��ʱNH3����������� ��
��
��4������ͼ���֪����25 minʱ�����������ʵ�����0.3mol���͵�0.2mol�����Ըı��������������0.1molNH3��
���㣺���鷴Ӧ���ʵļ��㡢ƽ�ⳣ�����жϡ����������ƽ��״̬��Ӱ���Լ��йؼ���
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������͡������ۺ���ǿ�����ض�ѧ��������������������ע�ػ���֪ʶ���̣����ض�ѧ�����ⷽ����ָ����ѵ���������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԡ�

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д���27�֣����ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��![]() 2NH3��g�� ����H=��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺
2NH3��g�� ����H=��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺

��1��10min����NH3��ʾ��ƽ����Ӧ���� ��
��2����10~20 min�ڣ�NH3Ũ�ȱ仯��ԭ������� ��
A�����˴��� B����С�������
C�������¶� D������NH3���ʵ���
��3����1��ƽ���ʱ�䷶ΧΪ�� ��
��2��ƽ���ʱ�䷶ΧΪ�� ��
��1��ƽ�⣺ƽ�ⳣ��K1 = �������ݵı���ʽ����
��2��ƽ��ʱNH3��������� ��
��4���ڷ�Ӧ������25 minʱ��
�� ���߷����仯��ԭ��
�� ��ڶ���ƽ��ʱ����ƽ���ƽ�ⳣ��
K2 K1������ڡ��������ڡ�����С�ڡ�����
��5����25~40minʱ������ϳɰ������еķ�Ӧ�ȡ�H= ��
��6���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ�������з�Ӧ��
N2��g��+ 3H2O��1�� ![]() 2NH3��g��+
2NH3��g��+ ![]() O2��g�� ����H = a kJ��mol��1
O2��g�� ����H = a kJ��mol��1
��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| T/K | 303 | 313 | 323 |
| NH3������/��10-6mol�� | 4��8 | 5��9 | 6��0 |
�ٴ˺ϳɷ�Ӧ��a 0����S 0�����������������������
����֪��N2��g��+ 3H2��g��![]() 2NH3��g�� ��H= ��92 ��4kJ��mol��1
2NH3��g�� ��H= ��92 ��4kJ��mol��1
2H2��g��+ O2��g�� = 2H2O��l��= ��571��6kJ��mol��1
�����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ��
��17�֣����ĺϳ�ԭ��Ϊ��N2��g��+3H2��g�� 2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ��
2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ��
�ش��������⣺
��1��10 min����NH3��ʾ��ƽ����Ӧ���� ��
��2����10 ~20 min�ڣ�NH3Ũ�ȱ仯��ԭ������� ��
| A�����˴��� | B����С������� | C�������¶� | D������NH3���ʵ��� |
��1��ƽ�⣺ƽ�ⳣ��K1 = �������ݵı���ʽ������2��ƽ��ʱNH3��������� ��
��4���ڷ�Ӧ������25 minʱ��
�����߷����仯��ԭ��
�ڴ�ڶ���ƽ��ʱ����ƽ���ƽ�ⳣ��K2 K1������ڡ��������ڡ�����С�ڡ�����
��5����25~40 minʱ������ϳɰ������еķ�Ӧ�ȡ�H= ��
��6���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ�������з�Ӧ��
N2��g�� + 3H2O��1��
 2NH3��g�� +
2NH3��g�� +  O2��g�� ����H =" a" kJ��mol��1
O2��g�� ����H =" a" kJ��mol��1��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| T/K | 303 | 313 | 323 |
| NH3������/��10-6mol�� | 4��8 | 5��9 | 6��0 |
����֪��N2��g�� + 3H2��g��
 2NH3��g�� ��H= ��92 ��4kJ��mol��1
2NH3��g�� ��H= ��92 ��4kJ��mol��12H2��g�� + O2��g�� = 2H2O��l�� = ��571��6kJ��mol��1
�����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ��
 ���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4KJ?mol-1������500�桢20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ���ش��������⣺
���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4KJ?mol-1������500�桢20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ���ش��������⣺ 2NH3(g)
����H= ��92.4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺
2NH3(g)
����H= ��92.4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺