��Ŀ����
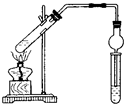 ����ͼ��ʾʵ��װ����ȡ�����������ش��������⣺
����ͼ��ʾʵ��װ����ȡ�����������ش��������⣺��1���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�� �����ǣ������Թ��м���һ������
�Ҵ�
�Ҵ�
��Ȼ�����������Ũ����
Ũ����
����ȴ���ټ���һ������������
������
��������ʹ֮��Ͼ��ȣ���2��Ũ����������ǣ�
��������ˮ��
��������ˮ��
����3������̼������Һ�������ǣ���
���������������ܽ��
���������������ܽ��
���������Ҵ�
�����Ҵ�
���������
�����
����4�����ɵ��������������ܶȱ�ˮ
С
С
���������������
��
ζ����5����ʵ���з�Ӧ�¶Ȳ�����ʹ��Ӧ�¶�ά����140�����ң���ʱ����Ӧ����Ҫ�л�����Ľṹ��ʽΪ��
CH3CH2OCH2CH3
CH3CH2OCH2CH3
����6�������в��õ�ʵ��װ�õIJ�֮ͬ���ǣ���ʵ����������θ���ܴ����˳����ܣ���������ܵ�ĩ�˲����˱���̼������Һ�У��ڴ˴����θ���ܵ������У�
��
��ֹ����
��ֹ����
����ʹ��������Һ��ֽӴ�
ʹ��������Һ��ֽӴ�
����������1��Ϊ��ֹ��Һ�ɽ���Ӧ�ȼ����Ҵ���Ȼ���ڼ���Ũ��������
��2���������Ҵ���Ũ���������¼��ȷ���������Ӧ���÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���
��3���Ʊ���������ʱ���ñ���̼������Һ������������������̼������Һ����Ҫ�������ܽ��Ҵ���̼���������ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻
��4��������������Һ�ϲ㣬��һ������ζ��Һ�壻
��5���������Ҵ���Ũ���������¼���140�����ҷ���ȡ����Ӧ���������ѣ�
��6�����θ�����ݻ��ϴ���ֹ���������ã��������������������Һ�Ӵ���
��2���������Ҵ���Ũ���������¼��ȷ���������Ӧ���÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���
��3���Ʊ���������ʱ���ñ���̼������Һ������������������̼������Һ����Ҫ�������ܽ��Ҵ���̼���������ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻
��4��������������Һ�ϲ㣬��һ������ζ��Һ�壻
��5���������Ҵ���Ũ���������¼���140�����ҷ���ȡ����Ӧ���������ѣ�
��6�����θ�����ݻ��ϴ���ֹ���������ã��������������������Һ�Ӵ���
�����1��Ϊ��ֹ��Һ�ɽ���Ӧ�ȼ����Ҵ���Ȼ���ڼ���Ũ��������ᣬ���ȼ�Ũ����������Һ�ɽ��Ŀ�������
�ʴ�Ϊ���Ҵ���Ũ��������
��2���������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ��������ˮ����
�ʴ�Ϊ����������ˮ����
��3���Ʊ���������ʱ���ñ���̼������Һ������������������̼������Һ����Ҫ�������ܽ��Ҵ���̼���������ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻�ʴ�Ϊ�����������������ܽ�ȣ������Ҵ����к����
��4��������������Һ�ϲ㣬�����ܶȱ�ˮС����һ������ζ��Һ�壻�ʴ�Ϊ��С���㣻
��5���������Ҵ���Ũ���������¼���140�����ҷ���ȡ����Ӧ���������ѣ���ṹ��ʽΪ��CH3CH2OCH2CH3���ʴ�Ϊ��CH3CH2OCH2CH3��
��6�����θ��������ϴ���ʹ��������Һ��ֽӴ���A�Թ����Ȳ��������θ���ܵĵ��ܿ�����Һ���¿��ܷ���������
�ʴ�Ϊ����ֹ������ʹ��������Һ��ֽӴ���
�ʴ�Ϊ���Ҵ���Ũ��������
��2���������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ��������ˮ����
�ʴ�Ϊ����������ˮ����
��3���Ʊ���������ʱ���ñ���̼������Һ������������������̼������Һ����Ҫ�������ܽ��Ҵ���̼���������ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻�ʴ�Ϊ�����������������ܽ�ȣ������Ҵ����к����
��4��������������Һ�ϲ㣬�����ܶȱ�ˮС����һ������ζ��Һ�壻�ʴ�Ϊ��С���㣻
��5���������Ҵ���Ũ���������¼���140�����ҷ���ȡ����Ӧ���������ѣ���ṹ��ʽΪ��CH3CH2OCH2CH3���ʴ�Ϊ��CH3CH2OCH2CH3��
��6�����θ��������ϴ���ʹ��������Һ��ֽӴ���A�Թ����Ȳ��������θ���ܵĵ��ܿ�����Һ���¿��ܷ���������
�ʴ�Ϊ����ֹ������ʹ��������Һ��ֽӴ���
���������⿼�������������Ʊ�����Ŀ�ѶȲ���ע�ⱥ��̼������Һ�������Լ�������Ӧ��ԭ����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
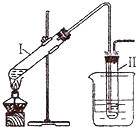 �������ʵ�飺������ͼ��ʾ��װ����ȡ�����飻�ڽ��������������ʵ�飮���Թ�I�����μ���2mL ����ˮ��4mLŨ���ᡢ2mL 95%���Ҵ���3g�廯�Ʒ�ĩ�����Թܢ���ע������ˮ�����ձ���ע������ˮ�������Թ�I����״̬�����Ӻ���ȴ��
�������ʵ�飺������ͼ��ʾ��װ����ȡ�����飻�ڽ��������������ʵ�飮���Թ�I�����μ���2mL ����ˮ��4mLŨ���ᡢ2mL 95%���Ҵ���3g�廯�Ʒ�ĩ�����Թܢ���ע������ˮ�����ձ���ע������ˮ�������Թ�I����״̬�����Ӻ���ȴ��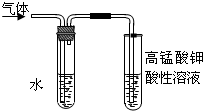
 ʵ���ҿ�������ͼ��ʾ��װ����ȡ�����������ش��������⣺
ʵ���ҿ�������ͼ��ʾ��װ����ȡ�����������ش��������⣺ ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�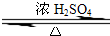 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O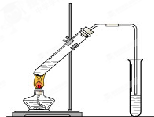 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺ CH3CO18OCH2CH3+H2O
CH3CO18OCH2CH3+H2O