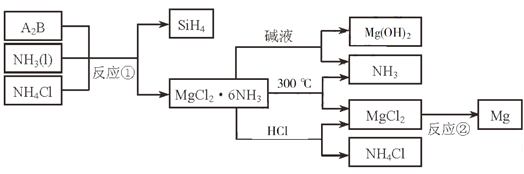
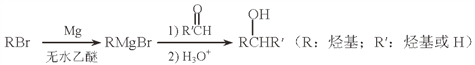
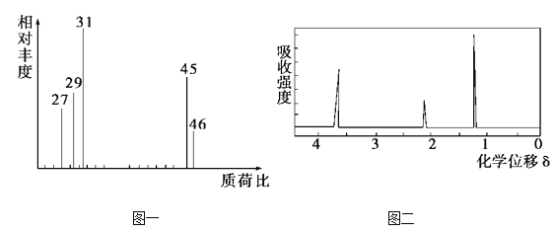
��Ŀ����
����Ŀ��(1)�����Ľṹ��ͼ1��ʾ�����ɿ��������ĸ���ͬ����Ԫ����ɵĿռ乹�͡�
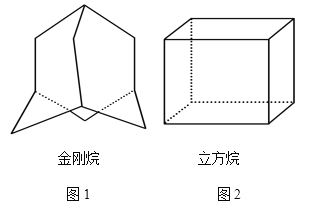
�������ķ���ʽΪ________��
��������ѧѧ����ͬ���칹���жϹ����ж�����ԭ��ȡ�������е���ԭ���γɵ�һ�������_____�֡�
(2)������������һ���ºϳɵ����������Ϊ������ṹ����̼�ܽṹ��ͼ2��ʾ��
��������ķ���ʽΪ______________________��
����������Ķ��ȴ������ͬ���칹�����Ŀ��_____________��
����a g����������b mL���У�Ȼ��ͨ��c L��Ȳ(��״����)�����û������̼�İٷֺ���Ϊ_______________��
���𰸡�C10H16 2 C8H8 3 92.3%
��������
(1)�ٸ��ݽ����Ľṹ������
�ڽ������һ�ָ߶ȶԳƽṹ��������������̼ԭ��(�Ǽ�̼ԭ�Ӻʹμ�̼ԭ��)��
(2)�ٸ���������Ľṹ������
�ڸ���������������������λ��ֻ��3�������
�۸��������顢������Ȳ���ߵķ���ʽ������
(1)�ٸ��ݽ����Ľṹ��֪������ʽΪC10H16���ʴ�Ϊ��C10H16��
�ڽ������һ�ָ߶ȶԳƽṹ��������������̼ԭ��(�Ǽ�̼ԭ�Ӻʹμ�̼ԭ��)�����һ�������2�֣��ʴ�Ϊ��2��
(2)�ٸ���������Ľṹ��֪������ʽΪC8H8���ʴ�Ϊ��C8H8��
�ڸ���������������������λ��ֻ��3����������Զ��ȴ���Ӧ��3�ֽṹ���ʴ�Ϊ��3��
�������顢������Ȳ���ߵ�ʵ��ʽ��Ϊ��CH���������κεı�ֵ��ϣ������û������̼�İٷֺ�����һ�����ٷֺ���Ϊ![]() =92.3%���ʴ�Ϊ��92.3%��
=92.3%���ʴ�Ϊ��92.3%��

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�����Ŀ��I.�������������������������ܹ������練Ӧ��2MnO4��+5H2C2O4+6H��=2Mn2��+10CO2��+8H2O���� 4mL 0.001mol/L KMnO4 ��Һ��2mL 0.01mol/L H2C2O4 ��Һ���о���ͬ�����Ի�ѧ��Ӧ���ʵ�Ӱ�죮�ı���������£�
��� | 10%�������/mL | �¶�/�� | �������� |
�� | 2mL | 20 | |
�� | 2mL | 20 | 10 �α��� MnSO4 ��Һ |
�� | 2mL | 30 | |
�� | 1mL | 20 | 1mL ����ˮ |
��1���÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ_____
��2������о������Ի�ѧ��Ӧ���ʵ�Ӱ�죬ʹ��ʵ��_________��_____������������ʾ����ͬ���� ����о��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬ʹ��ʵ��_____��_________��
��3���Ա�ʵ���������������о�_____�Ի�ѧ��Ӧ���ʵ�Ӱ�죬ʵ�����м��� 1mL����ˮ��Ŀ����_______
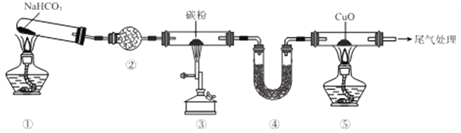
II��������ͼװ�ò����ʵ��Լ������ij̽��ʵ�飬���ó���Ӧʵ����ۣ�����������Ϣ�ش�

��1��Ϊ��֤��Ԫ�صķǽ�����ǿ���� S��C��Si������Ϊ������Ӧ���ǣ� ��Ϊ______����Ϊ______����Ϊ______������֪���������ݲ��������� �а�ɫ������
��2�������Ϊˮ����Ϊ Na2O2 ��ĩ����Ϊ H2S �ı���ˮ��Һ��ʵ���й۲쵽�������ɵ���ɫ������˵��Ԫ�أϡ��ӵõ�������ǿ��Ϊ ______��
��3������װ�����Ӻú��ڼ���ҩƷ��ʼʵ��ǰ������������Լ�飬��������ò���_____��