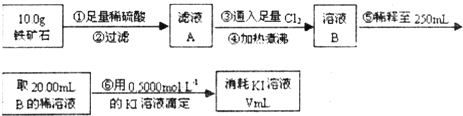
题目内容
(15分)Ⅰ某研究性学习小组对过量炭粉与氧化铁反应的气体产物成分进行研究。
(1)提出假设:①该反应的气体产物是CO2。②该反应的气体产物是CO。③该反应的气体产物是 
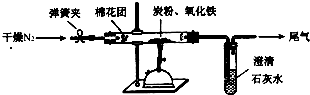
(2)设计方案:如图所示,将一定量的氧化铁在隔绝空气的条件下与过量炭粉完全反应,测定参加反应的碳元素与氧元素的质量比。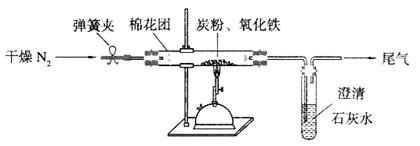
(3)查阅资料:氮气不与碳、氧化铁发生反应。实验室可以用氯化铵饱和溶液和亚硝酸钠(NaNO2)饱和溶液混合加热反应制得氮气。
①请写出该反应的离子方程式: 。
②下列装置可用于实验室制取氮气的是 。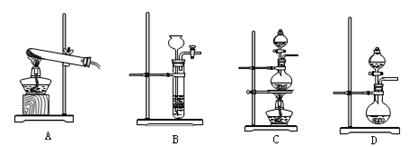
(4)实验操作及实验现象:
①按上图连接装置,并检查装置的气密性,称取3.20g氧化铁与2.00g炭粉混合均匀,放入48.48g的硬质玻璃管中;
②加热前,先通一段时间纯净干燥的氮气;
③停止通入N2后,夹紧弹簧夹,加热一段时间,澄清石灰水变浑浊;
④待反应结束,再通一段时间的氮气。冷却至室温,称得硬质玻璃管和固体总质量为52.24g。
(5)数据处理,经计算,参加反应的碳元素质量为0.48g,氧元素为0.96g。推断假设 成立。 该实验中发生的化学方程式为 。
该实验中发生的化学方程式为 。
(6)实验优化:根据实验得出的结论,应对该实验装置进一步完善,你认为应作如何改进? 。
Ⅱ由碳的氧化物直接合成甲醇、乙醇燃料已进入工业生产。如:
反应① CO(g)+2H2(g)  CH3OH(g)
CH3OH(g)
反应② 2CO(g)+4H2 (g) CH3CH2OH (g)+H2O (g)
CH3CH2OH (g)+H2O (g)
某同学为了寻找合成甲醇的适宜条件[温度、压强、碳氢比n(CO)/n(H2)、催化剂质量],设计了如下对比实验,部分实验条件已经填在下面实验设计表中(每次实验,反应时间相同)。
| 实验编号 | T(℃) | n (CO)/n(H2) | P(MPa) | CO转化率(%) |
| 1 | 150 | 1/3 | 0.1 | K^S*5U.C# |
| 2 | x | 1/3 | 5 | |
(2)若要探究温度、压强、碳氢比n(CO)/n(H2)、催化剂质量对合成甲醇的影响,除实验1、2外,至少还需进行 次对比实验。
(3)上述合成甲醇、乙醇的两个反应更符合绿色化学理念的是反应 (填编号),另一反应的平衡常数表达式为 。 K^S*5U.C#
Ⅰ(1)CO2、CO的混合物(1分)
(3)NH4++NO2- N2↑+2H2O(2分)C(1分)
N2↑+2H2O(2分)C(1分)
(5)③(2分)2C+Fe2O3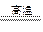 2Fe+CO↑+CO2↑(2分)
2Fe+CO↑+CO2↑(2分)
(6)在尾气出口处加一点燃的酒精灯或增加一尾气处理装置(2分,其他合理答案也给分)
Ⅱ(1)压强(1分)150(1分)(2)3(1分)
(3)①(1分)K= (1分)
(1分)
解析

 时刻准备着暑假作业原子能出版社系列答案
时刻准备着暑假作业原子能出版社系列答案 暑假衔接教材期末暑假预习武汉出版社系列答案
暑假衔接教材期末暑假预习武汉出版社系列答案 某研究性学习小组对铝热反应实验展开研究.现行高中化学教材中对“铝热反应”的现象有这样的描述:“反应放出大量的热,并发出耀眼的光芒”、“纸漏斗的下部被烧穿,有熔融物落入沙中”.查阅《化学手册》知,Al、Al2O3、Fe、Fe2O3熔点、沸点数据如下:
某研究性学习小组对铝热反应实验展开研究.现行高中化学教材中对“铝热反应”的现象有这样的描述:“反应放出大量的热,并发出耀眼的光芒”、“纸漏斗的下部被烧穿,有熔融物落入沙中”.查阅《化学手册》知,Al、Al2O3、Fe、Fe2O3熔点、沸点数据如下:| 物质 | Al | Al2O3 | Fe | Fe2O3 |
| 熔点/℃ | 660 | 2054 | 1535 | 1462 |
| 沸点/℃ | 2467 | 2980 | 2750 | -- |
(2)设计一个简单的实验方案,证明上述所得的块状熔融物中含有金属铝.该实验所用试剂是
(3)实验室溶解该熔融物,下列试剂中最好的是
A.浓硫酸 B.稀硫酸 C.稀硝酸 D.氢氧化钠溶液
Ⅱ.实验研究发现,硝酸发生氧化还原反应时,硝酸的浓度越稀,对应还原产物中氮元素的化合价越低.某同学取一定量上述的熔融物与一定量很稀的硝酸充分反应,反应过程中无气体放出.在反应结束后的溶液中,逐滴加入4mol?L-1的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示.则B点对应的沉淀的物质的量为


 某研究性学习小组对过量炭粉与氧化铁反应的气体产物成分进行研究.
某研究性学习小组对过量炭粉与氧化铁反应的气体产物成分进行研究.