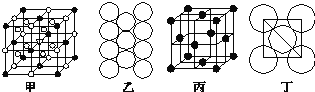
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ”–ΜζΈοFΒΡΚœ≥…¬ΖœΏ»γΆΦΥυ ΨΘΚ

“―÷Σ“‘œ¬–≈œΔΘΚ
Θ®1Θ©RCOORΓ·![]() RCH2OH
RCH2OH
Θ®2Θ©
ΜΊ¥π“‘œ¬Έ ΧβΘΚ
Θ®1Θ©AΒΡΫαΙΙΦρ ΫΈΣ Θ§GΒΡΫαΙΙΦρ ΫΈΣ ΓΘ
Θ®2Θ©B…ζ≥…CΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
Θ®3Θ©Φλ―ιE÷–Κ§―θΙΌΡήΆ≈ΒΡ ‘ΦΝ « Θ§
Ζ¥”Πœ÷œσ « ΓΘ
Θ®4Θ©EΓζFΒΡΖ¥”Πάύ–Ά « ΓΘ
Θ®5Θ©AΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§ΖϊΚœœ¬Ν–ΧθΦΰΒΡΆ§Ζ÷“λΙΙΧε”– ÷÷(≤ΜΑϋΚ§A)Θ§–¥≥ωΤδ÷–“Μ÷÷ΒΡΫαΙΙΦρ Ϋ ΓΘ
ΔΌ τ”ΎΖΦœψΉεΜ·ΚœΈο
ΔΎ±ΫΜΖ…œ”–ΥΡΗω»Γ¥ζΜυ
Δέ±ΫΜΖ…œΒΡ“Μδε»Γ¥ζΈο÷Μ”–“Μ÷÷
ΓΨ¥πΑΗΓΩΘ®1Θ©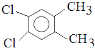 ΘΜ
ΘΜ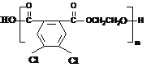 ΘΜ
ΘΜ
Θ®2Θ©![]() +2 CH3OH
+2 CH3OH![]()
![]() +2H2OΘΜ
+2H2OΘΜ
Θ®3Θ©“χΑ±»ή“ΚΜρ–¬÷Τ«β―θΜ·Ά≠–ϋΉ«“ΚΘΜ ‘Ιή±ΎΗΫΉ≈ΙβΝΝΒΡ“χΜρ≥ωœ÷Ή©Κλ…Ϊ≥ΝΒμΘΜ
Θ®4Θ©Φ”≥…Ζ¥”ΠΘΜ
Θ®5Θ©5ΘΜ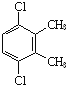 (Μρ
(Μρ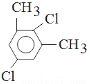 ΓΔ
ΓΔ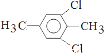 ΓΔ
ΓΔ![]() ΓΔ
ΓΔ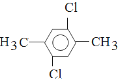 )ΓΘ
)ΓΘ
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΗυΨίΚœ≥…Νς≥ΧΩ…÷ΣΘ§AΡή±ΜΥα–‘ΗΏΟΧΥαΦΊ»ή“Κ―θΜ·…ζ≥…2Ηωτ»ΜυΘ§ΫαΚœ“―÷Σ–≈œΔΩ…÷ΣA”ΠΗΟΚ§”–2ΗωΦΉΜυΘΜΗυΨί”–ΜζΈοFΒΡΫαΙΙΦρ ΫΩ…÷ΣA÷–ΒΡ2ΗωΦΉΜυ”ΠΗΟ «ΝΎΈΜΒΡΘ§‘ρAΒΡΫαΙΙΦρ ΫΈΣΘΜ![]() Θ§‘ρBΒΡΫαΙΙΦρ ΫΈΣΘΚ
Θ§‘ρBΒΡΫαΙΙΦρ ΫΈΣΘΚ![]() ΘΜBΚΆΦΉ¥ΦΖΔ…ζθΞΜ·Ζ¥”Π…ζ≥…CΘ§‘ρCΒΡΫαΙΙΦρ ΫΈΣΘΚ
ΘΜBΚΆΦΉ¥ΦΖΔ…ζθΞΜ·Ζ¥”Π…ζ≥…CΘ§‘ρCΒΡΫαΙΙΦρ ΫΈΣΘΚ![]() ΘΜBΖ÷Ή”÷–Κ§”–2Ηωτ»ΜυΘ§”κ““Εΰ¥ΦΖΔ…ζΥθΨέΖ¥”Π…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοGΘ§‘ρGΒΡΫαΙΙΦρ ΫΈΣΘΚ
ΘΜBΖ÷Ή”÷–Κ§”–2Ηωτ»ΜυΘ§”κ““Εΰ¥ΦΖΔ…ζΥθΨέΖ¥”Π…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοGΘ§‘ρGΒΡΫαΙΙΦρ ΫΈΣΘΚ ΘΜΗυΨί“―÷Σ–≈œΔΩ…÷ΣC…ζ≥…DΘ§‘ρDΒΡΫαΙΙΦρ ΫΈΣΘΚ
ΘΜΗυΨί“―÷Σ–≈œΔΩ…÷ΣC…ζ≥…DΘ§‘ρDΒΡΫαΙΙΦρ ΫΈΣΘΚ![]() Θ§DΖΔ…ζ¥ΏΜ·―θΜ·…ζ≥…EΘ§‘ρEΒΡΫαΙΙΦρ ΫΈΣ
Θ§DΖΔ…ζ¥ΏΜ·―θΜ·…ζ≥…EΘ§‘ρEΒΡΫαΙΙΦρ ΫΈΣ![]() Θ§E‘ΎΥΪ―θΥ°ΒΡΉς”Οœ¬…ζ≥…FΘ§
Θ§E‘ΎΥΪ―θΥ°ΒΡΉς”Οœ¬…ζ≥…FΘ§
Θ®1Θ©Ά®Ιΐ“‘…œΖ÷Έω÷ΣΘ§AΓΔGΒΡΫαΙΙΦρ ΫΖ÷±πΈΣ ΓΔ
ΓΔ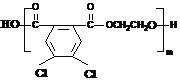 Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ ΘΜ
ΘΜ ΘΜ
ΘΜ
Θ®2Θ©BΚΆΦΉ¥Φ‘Ύ≈®ΝρΥαΉς¥ΏΜ·ΦΝΓΔΦ”»»ΧθΦΰœ¬ΖΔ…ζθΞΜ·Ζ¥”Π…ζ≥…CΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣ![]() +2 CH3OH
+2 CH3OH![]()
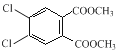 +2H2OΘ§Ι ¥πΑΗΈΣΘΚ
+2H2OΘ§Ι ¥πΑΗΈΣΘΚ![]() +2 CH3OH
+2 CH3OH![]()
![]() +2H2OΘΜ
+2H2OΘΜ
Θ®3Θ©EΒΡΫαΙΙΦρ ΫΈΣ![]() Θ§Τδ÷–Κ§―θΙΌΡήΆ≈ «»©ΜυΘ§Ω…“‘”Ο“χΑ±»ή“ΚΜρ–¬÷Τ«β―θΜ·Ά≠–ϋΉ«“ΚΦλ―ι»©ΜυΘ§Τδœ÷œσ « ‘Ιή±ΎΗΫΉ≈ΙβΝΝΒΡ“χΜρ≥ωœ÷Ή©Κλ…Ϊ≥ΝΒμΘ§Ι ¥πΑΗΈΣΘΚ“χΑ±»ή“ΚΜρ–¬÷Τ«β―θΜ·Ά≠–ϋΉ«“ΚΘΜ ‘Ιή±ΎΗΫΉ≈ΙβΝΝΒΡ“χΜρ≥ωœ÷Ή©Κλ…Ϊ≥ΝΒμΘΜ
Θ§Τδ÷–Κ§―θΙΌΡήΆ≈ «»©ΜυΘ§Ω…“‘”Ο“χΑ±»ή“ΚΜρ–¬÷Τ«β―θΜ·Ά≠–ϋΉ«“ΚΦλ―ι»©ΜυΘ§Τδœ÷œσ « ‘Ιή±ΎΗΫΉ≈ΙβΝΝΒΡ“χΜρ≥ωœ÷Ή©Κλ…Ϊ≥ΝΒμΘ§Ι ¥πΑΗΈΣΘΚ“χΑ±»ή“ΚΜρ–¬÷Τ«β―θΜ·Ά≠–ϋΉ«“ΚΘΜ ‘Ιή±ΎΗΫΉ≈ΙβΝΝΒΡ“χΜρ≥ωœ÷Ή©Κλ…Ϊ≥ΝΒμΘΜ
Θ®4Θ©Ά®Ιΐ“‘…œΖ÷Έω÷ΣΘ§EΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…FΘ§Υυ“‘ΤδΖ¥”Πάύ–Ά «Φ”≥…Ζ¥”ΠΘ§Ι ¥πΑΗΈΣΘΚΦ”≥…Ζ¥”ΠΘΜ
Θ®5Θ©ΔΌ τ”ΎΖΦœψΉεΜ·ΚœΈοΥΒΟςΚ§”–±ΫΜΖΘ§ΔΎ±ΫΜΖ…œ”–ΥΡΗω»Γ¥ζΜυ«“Δέ±ΫΜΖ…œΒΡ“Μδε»Γ¥ζΈο÷Μ”–“Μ÷÷Θ§‘ρΥΒΟς»Γ¥ζΜυ‘Ύ±ΫΜΖ…œΒΡΈΜ÷Ο”ΠΗΟ «Ε‘≥ΤΒΡΘ§Υυ“‘ΖϊΚœΧθΦΰΒΡAΒΡΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ Ϋ”–ΘΚ ΓΔ
ΓΔ ΓΔ
ΓΔ ΓΔ
ΓΔ![]() ΓΔ
ΓΔ Θ§ΉήΙ≤”–5÷÷Θ§Ι ¥πΑΗΈΣΘΚ5ΘΜ
Θ§ΉήΙ≤”–5÷÷Θ§Ι ¥πΑΗΈΣΘΚ5ΘΜ (Μρ
(Μρ ΓΔ
ΓΔ ΓΔ
ΓΔ![]() ΓΔ
ΓΔ )ΓΘ
)ΓΘ