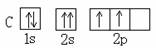
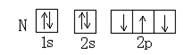
��Ŀ����
��8�֣��±���Ԫ�����ڱ���ǰ������ȥ�������Ϸ��Ŀհ��������϶��ɣ��������ߴ�Ϊ��A����A������Ӵ���������Ӧ�Ļ�ѧ�������Żش��������⣺

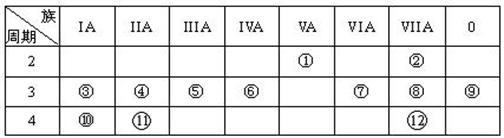
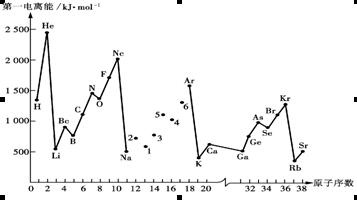
��1����ͼ��1���DZ�ʾ��������8��Ԫ�ص��ʵ��۵㣨�棩����ͼ����֪���Ρ�1������Ar�����������Ρ�8���������� ���壨�������ͣ���
��2��b��c��d��e��f���⻯��ķе㣨�棩ֱ������ͼ��2�������С�5�����⻯���������
�����С�2�����⻯��ĽṹʽΪ ��
��3��eԪ����fԪ����ȣ��縺��f����e�����б�������֤����һ��ʵ���� ����ѡ����ţ�
A��������f���ʵ���ɫ��e���ʵ���ɫ��
B��f������e���⻯����ҷ�Ӧ������e�ĵ���
C��f��e�γɵĻ�������eԪ�س�����̬
D���Ƚ���Ԫ�صĵ�������������ʱ�õ��ӵ���Ŀ
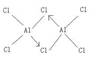
��4����ѧ��֤ʵ���Ȼ������ڹ��ۻ��������ʽΪAl2Cl6����ÿ��Ԫ�ؾ�����8���ӵĽṹ����д������Ϊ��ȷ��Al2Cl6�ṹ��_______________��
��5������������һ����Ҫ�������ըҩ�����Ի������о�����Ԫ�زⶨ����Խ��Խ�������ǵ����ӣ��ɵ�������(Sodium azida)NaN3�ȷֽ�ɵù��״�N2��2NaN3(s)��2Na(l)+3N2(g)���й�˵����ȷ����_____________(ѡ�����)

A���ƾ����ṹ��ͼ���ƾ�����ÿ����ԭ�ӵ���λ��Ϊ6
B���ƾ����ṹ��ͼ�������з�̯2����ԭ��
C�����ĵ縺�Դ�����
D��Na+�İ뾶С��N3-�İ뾶
| a | | | | | | | |
| b | | | c | d | e | f | |
| g | | h | | | | | |

��1����ͼ��1���DZ�ʾ��������8��Ԫ�ص��ʵ��۵㣨�棩����ͼ����֪���Ρ�1������Ar�����������Ρ�8���������� ���壨�������ͣ���
��2��b��c��d��e��f���⻯��ķе㣨�棩ֱ������ͼ��2�������С�5�����⻯���������
�����С�2�����⻯��ĽṹʽΪ ��
��3��eԪ����fԪ����ȣ��縺��f����e�����б�������֤����һ��ʵ���� ����ѡ����ţ�
A��������f���ʵ���ɫ��e���ʵ���ɫ��
B��f������e���⻯����ҷ�Ӧ������e�ĵ���
C��f��e�γɵĻ�������eԪ�س�����̬
D���Ƚ���Ԫ�صĵ�������������ʱ�õ��ӵ���Ŀ
��4����ѧ��֤ʵ���Ȼ������ڹ��ۻ��������ʽΪAl2Cl6����ÿ��Ԫ�ؾ�����8���ӵĽṹ����д������Ϊ��ȷ��Al2Cl6�ṹ��_______________��
��5������������һ����Ҫ�������ըҩ�����Ի������о�����Ԫ�زⶨ����Խ��Խ�������ǵ����ӣ��ɵ�������(Sodium azida)NaN3�ȷֽ�ɵù��״�N2��2NaN3(s)��2Na(l)+3N2(g)���й�˵����ȷ����_____________(ѡ�����)

A���ƾ����ṹ��ͼ���ƾ�����ÿ����ԭ�ӵ���λ��Ϊ6
B���ƾ����ṹ��ͼ�������з�̯2����ԭ��
C�����ĵ縺�Դ�����
D��Na+�İ뾶С��N3-�İ뾶
(1) ԭ�� (2) ���飬 H-O-H (3) B �� C
(4)
��5�� B��D
(4)

��5�� B��D
������Ŀ���Ԫ�����ڱ���������֪a��b��c��d��e��f��g��h�ֱ�ΪH��Li��C��N��O��F��Na��Al�����۷е�ߵ�ϵһ�㣺ԭ�Ӿ���>���Ӿ���>���Ӿ��壬����������SiΪԭ�Ӿ��壬��ΪSi��ͼ2�У��۷е���ߵĿ���Ϊ���Ӿ��壨�����⻯�����͵�ΪCH4����4��AlCl3����ǹ��ۻ����Cl����8�����ȶ��ṹ��AlΪ6�����ӣ�Al2Cl6�ж�����8���ӣ���Ȼ������λ������5����ͼ�п������ƾ�����ÿ��Na��Χ��8���ƣ�����λ��Ϊ8���������Ƶĸ����þ�̯����1+8��=2���縺�ԣ�ͬ���ڴ������ң�������N<O��Na+��N3-�ĵ��Ӳ�ṹ��ͬ���˵����Խ�࣬�뾶ԽС����Na+<N3-.����ѡBD��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
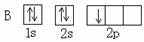
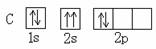 A B��
A B��