��Ŀ����
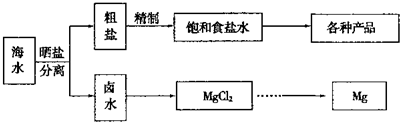
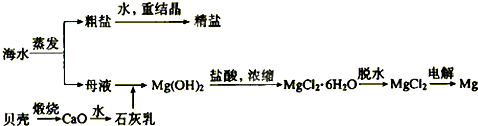
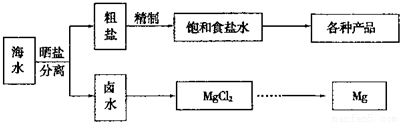
������ͼ��ˮ�ۺ����õĹ�ҵ����ͼ���ж�����˵����ȷ���ǣ�������
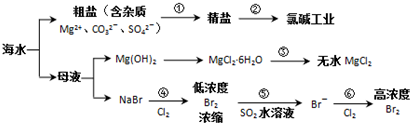
��֪��MgCl2?6H2O��������Mg��OH��Cl��HCl����ȣ�

��֪��MgCl2?6H2O��������Mg��OH��Cl��HCl����ȣ�
������A�����ݴ��ε��ᴿ����ⱥ��ʳ��ˮ��������жϣ�
B������þ����ˮ�⼰�Ȼ���Ļӷ��Խ��з�����
C���������ʵ�������������������������֪������Ħ�������
D�����ݷ�Ӧ�ݶ����������嵥�ʵ����ɲ�����з�����
B������þ����ˮ�⼰�Ȼ���Ļӷ��Խ��з�����
C���������ʵ�������������������������֪������Ħ�������
D�����ݷ�Ӧ�ݶ����������嵥�ʵ����ɲ�����з�����
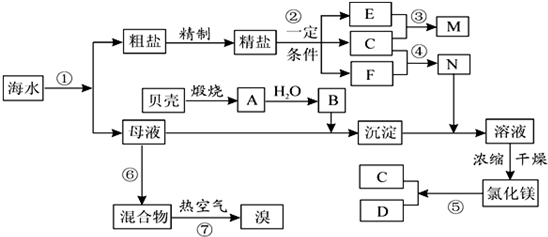
����⣺A�����̢ٵ��ᴿ�й��������������̣����dz�ȥþ���ӡ�̼������ӡ�������������ڻ�ѧ���̣����̢ڵ�ⱥ��ʳ��ˮ������������������ֵ��ʣ���A����
B����MgCl2?6H2O��������Mg��OH��Cl��HCl����ȣ��ò�����ˮMgCl2����Ҫ��MgCl2?6H2O���ռ����Ƶ���ˮMgCl2��Ϊ��ֹMg2+����ˮ�⣬Ӧ��HCl�����н��У���B����
C����2Br-+Cl2 =Br2 +2Cl-��ÿ����0.2molBr-������0.1molCl2��0.1molCl2�������״��Ϊ2.24L��������״���²�һ��Ϊ2.24L����C����
D����Br2+SO2 +2H2O=2HBr+H2SO4����Ӧ����Һ�����ԣ��������������Ӧ����D��ȷ��
��ѡ��D��
B����MgCl2?6H2O��������Mg��OH��Cl��HCl����ȣ��ò�����ˮMgCl2����Ҫ��MgCl2?6H2O���ռ����Ƶ���ˮMgCl2��Ϊ��ֹMg2+����ˮ�⣬Ӧ��HCl�����н��У���B����
C����2Br-+Cl2 =Br2 +2Cl-��ÿ����0.2molBr-������0.1molCl2��0.1molCl2�������״��Ϊ2.24L��������״���²�һ��Ϊ2.24L����C����
D����Br2+SO2 +2H2O=2HBr+H2SO4����Ӧ����Һ�����ԣ��������������Ӧ����D��ȷ��
��ѡ��D��
���������⿼���˺�ˮ��Դ�ۺ����ã��漰�����ӵij��ӡ�����ˮ�⡢������ԭ��Ӧ��Ԫ�ؼ��仯������й�֪ʶ�����ۺ����ã������������ۺ��ԣ������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ