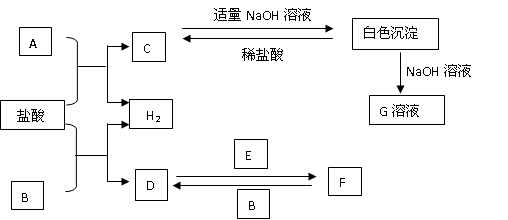
��Ŀ����
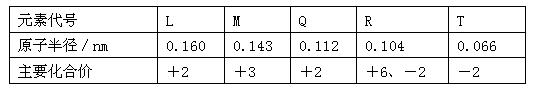
��12�֣��±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����ijһ��ѧԪ�ء�
(1)i����Ԫ�أ���ԭ�ӵ�����������Ϊ2����д����Ԫ�ص�ԭ�ӽṹʾ��ͼ__________��
(2)������Ԫ���γɵĽ��������У��۵���͵���__ __������Ԫ�ط��ţ�
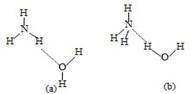
(3)NH3��H2O�ĵ��뷽��ʽΪNH3��H2O NH4++OH�������ж�NH3����ˮ����ͼ���γɵ�NH3��H2O�ĺ����ṹ��________������ţ���
NH4++OH�������ж�NH3����ˮ����ͼ���γɵ�NH3��H2O�ĺ����ṹ��________������ţ���
(4)aλ�ڵڢ�A�壬������ΪaҲ���Է��ڢ�A�壬����������֧�����ֹ۵����
(5)1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����_________����д��ĸ����
(6)����ϡ�����廯�������ʵ���������Ʊ�ʵ���ϵ�ͻ�ơ�����XeO3�ڼ�����������NaBrO3��Ӧ����NaBrO4��ͬʱ�ų�Xe��д���÷�Ӧ�ķ���ʽ��

(1)i����Ԫ�أ���ԭ�ӵ�����������Ϊ2����д����Ԫ�ص�ԭ�ӽṹʾ��ͼ__________��
(2)������Ԫ���γɵĽ��������У��۵���͵���__ __������Ԫ�ط��ţ�
(3)NH3��H2O�ĵ��뷽��ʽΪNH3��H2O
 NH4++OH�������ж�NH3����ˮ����ͼ���γɵ�NH3��H2O�ĺ����ṹ��________������ţ���
NH4++OH�������ж�NH3����ˮ����ͼ���γɵ�NH3��H2O�ĺ����ṹ��________������ţ���(4)aλ�ڵڢ�A�壬������ΪaҲ���Է��ڢ�A�壬����������֧�����ֹ۵����

| A��HF | B��H3O�� | C��NaH | D��H2O2 |
(6)����ϡ�����廯�������ʵ���������Ʊ�ʵ���ϵ�ͻ�ơ�����XeO3�ڼ�����������NaBrO3��Ӧ����NaBrO4��ͬʱ�ų�Xe��д���÷�Ӧ�ķ���ʽ��
(1) (2)e (3)(b) (4)C (5)j��(6)
(2)e (3)(b) (4)C (5)j��(6) 
 (2)e (3)(b) (4)C (5)j��(6)
(2)e (3)(b) (4)C (5)j��(6) 
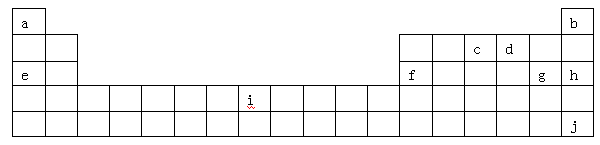
�������������Ԫ�������ڱ��еķֲ�������֪����a��H��b��He��c��N��d��O��e��Na��f��Al��g��Cl��h��Ar��i��Fe��j��Xe��
��1��Fe��26��Ԫ�أ�ԭ�ӽṹʾ��ͼΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2���ƺ����У��۵���͵���Na���ʴ�Ϊ��Na��
��3��NH3����ˮ���γɵ�NH3?H2O�У�����NH3?H2O�ĵ��뷽��ʽΪNH3?H2O
 NH4++OH-����֪�ṹ�к���笠����������Ļ����ṹ���ʴ�Ϊ����b����
NH4++OH-����֪�ṹ�к���笠����������Ļ����ṹ���ʴ�Ϊ����b������4�������ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ�ص���������ã���ϡ�����廯ѧ��������ȶ����������£�ϡ������Ԫ�ؽ�������ǿ�������˵��ʧȥ���ӣ����������Ƶ�j�ķ�������ʴ�Ϊ��j��
��5��������Ϣ��֪����Ӧ����XeO3��NaBrO3����������NaBrO4��Xe�����������ԭ���ӵ�ʧ��ƽ��֪��XeO3+3NaBrO3�T3NaBrO4+Xe���ʴ�Ϊ��XeO3+3NaBrO3�T3NaBrO4+Xe����
��6���Ȼ����Ǻ���H-Cl���ۼ��Ĺ��ۻ�����ʴ�Ϊ�����ۣ�
���������⿼��ѧ��Ԫ�����ڱ��еĻ���֪ʶ����Ŀ�����ѶȽϸߣ��ۺ��Խ�ǿ��

��ϰ��ϵ�д�
�����Ŀ

 Hԭ���е����Ӻ�����
Hԭ���е����Ӻ�����