��Ŀ����
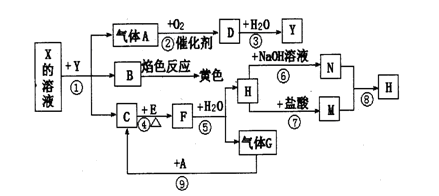
A��B��C��D��E����ѧ������5�ֻ����A��B�ǽ�����������A�Ǻ���ɫ���壬Ԫ��X��Y��Z����ѧ��ѧ�г����ĵ��ʣ�������ʼ�Ĺ�ϵ����ͼ��ʾ��
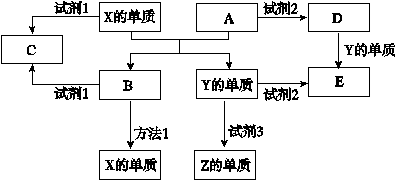
��1����ҵ�ϵõ�����X��ұ������1�� ��
��2������X���Լ�1������Y���Լ�2���ܲ���Z���ʣ��Լ�3�������г�����Һ�壬��д��Y���Լ�3 ��Ӧ�Ļ�ѧ����ʽ�� ��
��3�����Լ�1��NaOH��Һ������X���Լ�1��Ӧ�����ӷ���ʽ ��
��4�����Լ�2�����ᡣ
����μ���D�����еĽ��������� ��
�ڽ�D�ı�����Һ�μӵ���ˮ�еõ��ж����ЧӦ�ķ�ɢϵ�������ӷ���ʽ��ƽ���ƶ�����˵���������ַ�ɢϵ��ԭ�� ��

��1����ҵ�ϵõ�����X��ұ������1�� ��
��2������X���Լ�1������Y���Լ�2���ܲ���Z���ʣ��Լ�3�������г�����Һ�壬��д��Y���Լ�3 ��Ӧ�Ļ�ѧ����ʽ�� ��
��3�����Լ�1��NaOH��Һ������X���Լ�1��Ӧ�����ӷ���ʽ ��
��4�����Լ�2�����ᡣ
����μ���D�����еĽ��������� ��
�ڽ�D�ı�����Һ�μӵ���ˮ�еõ��ж����ЧӦ�ķ�ɢϵ�������ӷ���ʽ��ƽ���ƶ�����˵���������ַ�ɢϵ��ԭ�� ��
��9�֣�
��1����⣨1�֣�
��2��3Fe+4H2O��g��= Fe3O4+4H2��2�֣�
Fe3O4+4H2��2�֣�
��3��2Al+2OH-+2H2O==2AlO2 -+3H2����2�֣�
��4����ȡ����D��Һ���Թ��У������Թ��еμ����������軯����Һ������Һ�Ժ�ɫ����D�����еĽ�����������Fe3+����2�֣�
��D��Һ����Fe3++3H2O Fe(OH)3+3H+ˮ��ƽ�⣬������Ӧ�����ȷ�Ӧ������μӵ���ˮ��ʱʹƽ���������ƶ�������������Fe(OH)3����ɽ�����������ж����ЧӦ����2�֣�
Fe(OH)3+3H+ˮ��ƽ�⣬������Ӧ�����ȷ�Ӧ������μӵ���ˮ��ʱʹƽ���������ƶ�������������Fe(OH)3����ɽ�����������ж����ЧӦ����2�֣�
��1����⣨1�֣�
��2��3Fe+4H2O��g��=
 Fe3O4+4H2��2�֣�
Fe3O4+4H2��2�֣���3��2Al+2OH-+2H2O==2AlO2 -+3H2����2�֣�
��4����ȡ����D��Һ���Թ��У������Թ��еμ����������軯����Һ������Һ�Ժ�ɫ����D�����еĽ�����������Fe3+����2�֣�
��D��Һ����Fe3++3H2O
 Fe(OH)3+3H+ˮ��ƽ�⣬������Ӧ�����ȷ�Ӧ������μӵ���ˮ��ʱʹƽ���������ƶ�������������Fe(OH)3����ɽ�����������ж����ЧӦ����2�֣�
Fe(OH)3+3H+ˮ��ƽ�⣬������Ӧ�����ȷ�Ӧ������μӵ���ˮ��ʱʹƽ���������ƶ�������������Fe(OH)3����ɽ�����������ж����ЧӦ����2�֣����������A�ǽ�����������A�Ǻ���ɫ���壬��A����������A��X�����û���Ӧ����B��B�ǽ����������÷�Ӧ�������������������ȷ�Ӧ����X������Y������B�������������������������Լ�1��Ӧ����C���õ���������ķ���ұ������Al�����������������ᷴӦ������Ӧ����Fe���������������ᷴӦ�����Լ�1ΪNaOH��Һʱ��CΪƫ�����ƣ��Լ�2Ϊ����ʱ��DΪ�Ȼ�����EΪ�Ȼ�������
��1����ҵ���õ�������������ķ���ұ�������ʴ�Ϊ����⣻
��2������X���Լ�1������Y���Լ�2���ܲ���Z���ʣ���Z���������Լ�3�������г�����Һ�壬��Ϊˮ�������£�����ˮ������Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O��g��
 .Fe3O4+4H2��
.Fe3O4+4H2������3Fe+4H2O��g��
 Fe3O4+4H2��
Fe3O4+4H2����3�����Լ�1��NaOH��Һ����������������Һ��Ӧ����ƫ�����ƺ����������ӷ�Ӧ����ʽΪ��2Al+2OH��+2H2O�T2AlO2��+3H2�����ʴ�Ϊ��2Al+2OH��+2H2O�T2AlO2��+3H2����
��4�����Լ�2�����ᣬ
�������������ᷴӦ�����Ȼ����������Ӻ����軯����Һ��Ӧ����Ѫ��ɫ��Һ�����鷽��Ϊ��ȡ����D��Һ���Թ��У������Թ��еμ����������軯����Һ������Һ�Ժ�ɫ����D�����еĽ�����������Fe3����
��Ϊ��ȡ����D��Һ���Թ��У������Թ��еμ����������軯����Һ������Һ�Ժ�ɫ����D�����еĽ�����������Fe3����
���Ȼ�����ǿ�������Σ���ˮ�����������������������ˮ�ⷴӦ�����ȷ�Ӧ�������¶ȴٽ�����ˮ�⣬�Ӷ�����������Fe��OH��3����ɽ�����������ж����ЧӦ��
��Ϊ��D��Һ����Fe3��+3H2O
 Fe��OH��3+3H��ˮ��ƽ�⣬������Ӧ�����ȷ�Ӧ������μӵ���ˮ��ʱʹƽ���������ƶ�������������Fe��OH��3����ɽ�����������ж����ЧӦ��
Fe��OH��3+3H��ˮ��ƽ�⣬������Ӧ�����ȷ�Ӧ������μӵ���ˮ��ʱʹƽ���������ƶ�������������Fe��OH��3����ɽ�����������ж����ЧӦ������ ��������ƶϣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ