题目内容
向体积为Va的0.05 mol/L CH3COOH溶液中加入体积为Vb的0.05 mol/L KOH溶液,下列关系错误的是 ( )
| A.Va>Vb时:c(CH3COOH)+c(CH3COO-)>c(K+) |
| B.Va=Vb时:c(CH3COOH)+c(H+)=c(OH-) |
| C.Va<Vb时:c(CH3COO-)>c(K+)>c(OH-)>c(H+) |
| D.Va与Vb任意比时:c(K+)+c(H+)=c(OH-)+c(CH3COO-) |
C
若醋酸与氢氧化钾等体积混合,两者恰好完全反应生成醋酸钠和水;此时溶液中粒子关系为,电荷守恒:c(K+)+c(H+)=c(OH-)+c(CH3COO-),质量守恒:c(CH3COOH)+c(CH3COO-)=c(K+),质子守恒:c(OH-)= c(H+) +c(CH3COOH);所以
A正确,Va>Vb时:醋酸过量,即有c(CH3COOH)+c(CH3COO-)>c(K+);B正确,Va=Vb时:由质子守恒得c(CH3COOH)+c(H+)=c(OH-);C错,Va<Vb时:KOH过量,c(K+)>c(CH3COO-)>c(OH-)>c(H+);D正确,由电荷守恒可得,任意溶液中c(K+)+c(H+)=c(OH-)+c(CH3COO-);
A正确,Va>Vb时:醋酸过量,即有c(CH3COOH)+c(CH3COO-)>c(K+);B正确,Va=Vb时:由质子守恒得c(CH3COOH)+c(H+)=c(OH-);C错,Va<Vb时:KOH过量,c(K+)>c(CH3COO-)>c(OH-)>c(H+);D正确,由电荷守恒可得,任意溶液中c(K+)+c(H+)=c(OH-)+c(CH3COO-);

练习册系列答案
相关题目
 NH4Cl和0.1 mol·L
NH4Cl和0.1 mol·L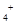 )>c(Cl
)>c(Cl )>c(Na
)>c(Na )>c(OH
)>c(OH )+c(HS
)+c(HS H3O+ + OH-
H3O+ + OH- (Na+)>c(H+)=c(OH-)
(Na+)>c(H+)=c(OH-) ②0.1mol/L
②0.1mol/L ③0.1mol/L
③0.1mol/L
 和0.1mol/L
和0.1mol/L 最小的是 ;
最小的是 ; 最小的是 (填序号)
最小的是 (填序号) ,则说明
,则说明 的水解程度 (填“>”、“<”或“=”)
的水解程度 (填“>”、“<”或“=”) 的水解程度,
的水解程度,
 (填“>”、“<”或“=”)
(填“>”、“<”或“=”)