��Ŀ����
��11�֣���ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ��������A����ij��̬���ʻ������ɻ�����B��
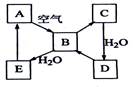
��1����������B������������Ҫ��Ⱦ�������D����ɵ���AԪ�ص�����������Ӧˮ�������A�� ���������ƣ�����������Bͨ����ˮ�й۲쵽�������� ��������˵��������B����
�ԣ�д��һ���ɻ�����D����B�Ļ�ѧ����ʽ ��
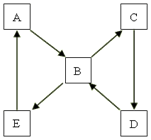
��2����������B����������ӡˢ��·�壬������B��E�����Ԫ����ͬ��������C����ɵ���AԪ�ص�����������Ӧˮ�������B��������ˮ����ԭ���� �������ӷ���ʽ��ʾ����ͬ����ӡˢ��·�������з�������Ҫ��Ӧ�� ������������Һ���Ƿ��л�����B��ʵ����������� ��
��1���ǣ�[1��]����ˮ��ɫ������ɫ��dz��[1��]����ԭ[1��]��
C+2H2SO4��Ũ��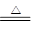 CO2��+2SO2��+2H2O[2��]����Cu��Na2SO3
+H2SO4��Ũ���ȴ𰸣�
CO2��+2SO2��+2H2O[2��]����Cu��Na2SO3
+H2SO4��Ũ���ȴ𰸣�
��2��Fe3++3H2O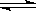 Fe(OH)3(����)+3H+[2��]��2Fe3++Cu=2Fe2++Cu2+[2��]��
Fe(OH)3(����)+3H+[2��]��2Fe3++Cu=2Fe2++Cu2+[2��]��
ȡ����[1��]������Һ���μ�KSCN��Һ[1��]���۲�����
����������1������������Ҫ��Ⱦ����SO2������B��SO2����A�ǵ���S��C����������D�����ᣬE�������������Ρ�SO2���л�ԭ�ԣ��ܱ���ˮ��������ʹ��ˮ��ɫ��Ũ�����ijЩ��ԭ�����ʷ�Ӧ���Ա���ԭ����SO2��
��2��������B����������ӡˢ��·�壬��B���Ȼ���������E���Ȼ�������A������C������������D�����������Ȼ�������Һ��ˮ�����������������壬���������ԣ�����ʽΪFe3++3H2O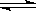 Fe(OH)3(����)+3H+���Ȼ�������������ͭ������ʽΪ2Fe3++Cu=2Fe2++Cu2+�����������ӿ���KSCN��Һ����ȡ����������Һ���μ�KSCN��Һ���۲�����
Fe(OH)3(����)+3H+���Ȼ�������������ͭ������ʽΪ2Fe3++Cu=2Fe2++Cu2+�����������ӿ���KSCN��Һ����ȡ����������Һ���μ�KSCN��Һ���۲�����