��Ŀ����
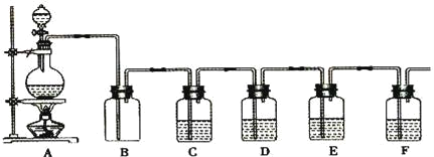
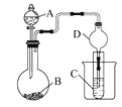
����Ŀ�����������ǹ����Ϲ��ϵĶԻ���������Ⱦ������ɱ��������������Ϊ���壬������ˮ�������ֽ⡣�״������Ʊ��������ȵĹ�����ͼ��
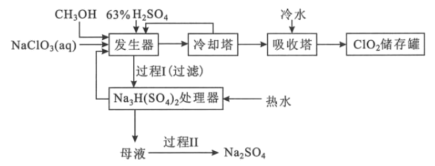
�ش��������⣺
��1��ClO2������ֽ��Ư�ס�ɱ���������������___�ԡ�
��2������������������Ũ������ϡ��Ϊ63%H2SO4����ȴ����뷴Ӧ����ԭ����___��ʵ�ʲ������������������м�������NaCl�Լӿ췴Ӧ���ʣ���NaCl�����ᷢ������Ⱦ������һ������ķ���ʽΪ___��
��3����1molCH3OH��Ӧʱת��6mole-���������������з�����Ӧ����Ҫ��ѧ����ʽΪ___��
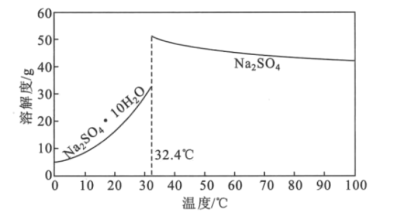
��4��Na2SO4��10H2O��Na2SO4���ܽ��������ͼ��ʾ���������̢����IJ����ǣ�����Ũ�����д�������������___���������Na2SO4���塣
��5�����ö��Ե缫Ϊ������ͨ���������������Һ�ķ���Ҳ�����Ʊ��������ȣ��������ĵ缫��ӦʽΪ___��
��6����״����Ʊ�����������ȣ���ⷨ���ŵ���___��д��2�㣩��
���𰸡�ǿ���� ������Ũ����ϡ�ͷ��ȣ�ʹClO2���ȷֽ� Cl2 CH3OH+6NaClO3+4H2SO4=2Na3H(SO4)2+CO2��+6ClO2��+5H2O �ڸ���32.4�������³��ȹ��ˡ�ϴ�� ClO2--e-=ClO2�� ��ɫ������������㣻ԭ�������ʸ�
��������
(1)ClO2����ǿ�����ԣ�
(2)Ũ�����ܽ���ˮ���ȣ���ClO2�����ֽ⣻Cl-�������������ܱ�ClO2������
(3)CH3OH��̼Ԫ�ػ��ϼ�Ϊ-2�ۣ���1molCH3OH��Ӧʱת��6mole-����CH3OH����������ΪCO2��
(4)���¶ȵ���32.4��ʱ��Na2SO4��10H2O���������Ͳ�Ʒ���ȣ�
(5)������ClO2-ʧ���ӷ���������Ӧ����ClO2��
(6)�״����������ӣ��ɱ��ߡ�
(1)����ClO2��ǿ�����ԣ�������ֽ��Ư�ס�ɱ��������
(2)Ũ������ϡ��Ϊ63%H2SO4������ȴ����뷴Ӧ���������IJ����ɱ�����Ũ����ϡ�ͷ��ȣ�ʹClO2���ȷֽ⣻��������NaCl�ɼӿ췴Ӧ���ʣ�������NaCl�����������£��ɱ�ClO2��������Cl2������Ⱦ������
(3)CH3OH��̼Ԫ�ػ��ϼ�Ϊ-2�ۣ���1molCH3OH��Ӧʱת��6mole-����CH3OH����������ΪCO2���������������з�����Ӧ����Ҫ��ѧ����ʽΪCH3OH+6NaClO3+4H2SO4=2Na3H(SO4)2+CO2��+6ClO2��+5H2O��
(4)���¶ȵ���32.4��ʱ��Na2SO4��10H2O�������������̢����м���Ũ�����д���������������Ҫ�ڸ���32.4�������³��ȹ��ˡ�ϴ�ӣ�����ٸ����Na2SO4���壻
(5)������ClO2-ʧ���ӷ���������Ӧ����ClO2���缫��ӦʽΪClO2--e-=ClO2����
(6)�Ƚϼ״������ⷨ����֪��ⷨ���ŵ�����ɫ������������㡢ԭ�������ʸߡ�