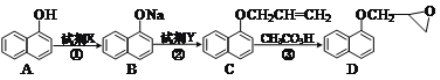
��Ŀ����
����Ŀ����1����֪��һ�������·�����Ӧ��A+B=C+D,14gA��9gBǡ����ȫ��Ӧ����0.5molC��1gD������C��Ħ������Ϊ____________;
��2��ij1L�����Һ���������ӵĸ��������±�����M���ӿ���Ϊ��_______��
�������� | NO3- | SO42- | Cu2+ | M |
������ | 4 | 1 | 2 | 1 |
A.Ba2+ B.Mg2+ C.Cl- D.Na+
��3����֪a g����X2�к���b��Xԭ�ӣ���ôc g�������ڱ�״���µ������____________;
��4����V L����MgSO4��K2SO4�Ļ����Һ�ֳ����ȷݣ�һ�ݼ��뺬a mol NaOH����Һ��ǡ��ʹþ������ȫ����ΪMg(OH)2����һ�ݼ��뺬b mol BaCl2����Һ��ǡ��ʹSO![]() ��ȫ����ΪBaSO4����ԭ�����Һ�м����ӵ����ʵ���Ũ��Ϊ________��
��ȫ����ΪBaSO4����ԭ�����Һ�м����ӵ����ʵ���Ũ��Ϊ________��
���𰸡�44 g/mol B 11.2cb/aNA ��4b-2a��/Vmol/L
��������
��1�����������غ��M=m/n���㣻
��2�����ݵ���ʻ����Һ��������������������ȷ��M����������ɣ�����������֮��ķ�Ӧ���жϴ��ڵ����ӣ�
��3����������������������Ħ�����������٤�������������������жϣ���Ҫ���ݹ�ϵΪ��n=m/M=N/NA=V/Vm��
��4�������Һ�ֳ����ȷݣ�ÿ����ҺŨ����ͬ��һ�ݼ��뺬a mol NaOH����Һ��ǡ��ʹþ������ȫת��ΪMg��OH��2����֪�÷���n��Mg2����=n[Mg��OH��2]=n��NaOH��/2����һ�ݼ��뺬bmol BaCl2����Һ��ǡ��ʹ�����������ȫ����ΪBaSO4�����ݱ�������������غ��֪�÷���n��SO42����=n��BaSO4��=n��BaCl2���������õ���غ��֪ÿ����2n��Mg2����+n��K����=2��SO42�������ݴ˼���ÿ����n��K����������c=n/V��������ӵ�Ũ�ȣ�
��1�����������غ�����C������Ϊ��14g+9g-1g=22g����M=m/n=22g/0.5mol=44g��mol��1��
��2����Һ�У���λ�������֪����������������Ϊ��2mol��L��1��2=4mol��L��1����λ�������֪�������������ܵ���Ϊ��4mol��L��1��1+1mol��L��1��2=6mol��L��1�����ڵ�λ�������֪����������������2mol��L��1����MΪ�����ӣ���M���ӵĵ��Ϊx���ɵ���غ��֪��6=4+x��1�����x=+2�����ѡ���֪��MΪBa2����Mg2������SO42����Ba2���ܽ���������ᱵ���������ܹ��棬����Һ�д��ڵ�����ΪMg2����
��ѡ��B��
��3��a g����X2�к���b��Xԭ�ӣ�����Ħ��������ΪM����ag/Mg��mol��1��2=b/NAmol��M=2aNA/bg��mol��1����״����c g����������=cg/Mg��mol��1��22.4L��mol��1= =
=![]() L��
L��
��4�������Һ�ֳ����ȷݣ�ÿ����ҺŨ����ԭ��ҺŨ����ͬ��һ�ݼ��뺬a mol NaOH����Һ��ǡ��ʹþ������ȫת��ΪMg��OH��2����֪�÷���n��Mg2����=n[Mg��OH��2]=n��NaOH��/2=a/2mol��
��һ�ݼ��뺬bmol BaCl2����Һ��ǡ��ʹ�����������ȫ����ΪBaSO4�����ݱ�������������غ��֪�÷���n��SO42����=n��BaSO4��=n��BaCl2��=bmol��
ÿ����Һ��2n��Mg2����+n��K����=2��SO42��������ÿ����Һ��n��K����=2��bmol-2��a/2mol=��2b-a��mol����ÿ����Һ�м����ӵ�Ũ��=(2b-a)mol/0.5VL=2(2b-a)/Vmol��L��1����ԭ��Һ�м����ӵ�Ũ��Ϊ2(2b-a)/Vmol��L��1��

 ��У����ϵ�д�
��У����ϵ�д�