��Ŀ����
ij�������λ�ѧʽΪXY������X+Ϊ�����ӣ�Y�DΪ�����ӣ�����һ�����ĸ�������������ˮ�С�
(1)�������Һ��pHΪ3���������Һ������Ũ�ȴ�С��ϵΪ��___________ ��
(2)�������Һ��pHΪ11���������ˮ��Ӧ�����ӷ���ʽΪ��_____________ ��
(3)�������Һ��pHΪ13����ȡ0.1mol/L��HY��Һ100mL�к͵�Ũ�ȵ�NaOH��Һ�����ԣ����� NaOH��Һ�����_____________100mL�������������������������
(1)�������Һ��pHΪ3���������Һ������Ũ�ȴ�С��ϵΪ��___________ ��
(2)�������Һ��pHΪ11���������ˮ��Ӧ�����ӷ���ʽΪ��_____________ ��
(3)�������Һ��pHΪ13����ȡ0.1mol/L��HY��Һ100mL�к͵�Ũ�ȵ�NaOH��Һ�����ԣ����� NaOH��Һ�����_____________100mL�������������������������
(1) c(Y-)��c(X+)��c(H+)��c(OH-)
(2) Y-+H2O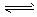 HY+OH-
HY+OH-
(3)��
(2) Y-+H2O
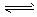 HY+OH-
HY+OH-(3)��

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ
 ��ˮ��Һ��pHΪ11��������ˮ��Ӧ�����ӷ���ʽΪ_______________________��
��ˮ��Һ��pHΪ11��������ˮ��Ӧ�����ӷ���ʽΪ_______________________��