��Ŀ����
����Ŀ��ij����С����Ƶ�ʵ������ȡ���ᴿ���������ķ���������ʾ
��֪:���Ȼ��ƿ����Ҵ��γ�CaCl26C2H5OH;
���й��л���ķе����±���ʾ
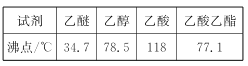
��2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
I.�Ʊ�����:װ����ͼ��ʾ��A��ʢ��Ũ���ᣬB��ʢ��9.5mL��ˮ�Ҵ���6mL�����ᣬD��ʢ�б���̼������Һ��
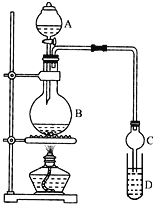
(1)ʵ������еμӴ�Լ3mLŨ���ᣬB���ݻ�����ʵ���_____(����ĸ����)
A. 25 mL B 50 mL C. 250 mL D. 500 mL
(2)���θ���ܵ���Ҫ������_______________��
(3)����̼������Һ��������______(����ĸ����)��
A.�������Ტ�ܽ��Ҵ�
B.̼������Һ�ʼ��ԣ�����������������ˮ��
C.�����������������ɣ���������
D.���������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�еĸ�С�������ڷֲ�����
II.�ᴿ����:
����D�л��Һ���з��롣
���л�����5mL����ʳ��ˮϴ�ӣ�����5mL�����Ȼ�����Һϴ�ӣ������ˮϴ�ӡ��л��㵹��һ�������ƿ�У�ѡ�ú��ʵĸ��������õ��ֲ�Ʒ��
�����ֲ�Ʒ�����ռ�77.1��ʱ����֣��õ����������������������
(4)������������Һʱ��ѡ�õ���Ҫ����������������_____________��
(5)���������ñ���ʳ��ˮϴȥ̼���ƺ����ñ����Ȼ�����Һϴ�ӣ���Ҫϴȥ�ֲ�Ʒ�е�______(����������)���ټ���_______(�˿մ�����ѡ����ѡ���������ʾ�����ˮ��)����
A.Ũ���� B.��ʯ�� C.��ˮ������ D.��ʯ��
(6)����������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ�һ�����ܵ�ԭ����_____________________________________________��
(7)��ʵ���������������Ϊ2.4g���Ҵ�������Ϊ2.1g���õ������IJ�Ʒ������Ϊ2.64g�������������IJ�����___________________��
���𰸡�B ��ֹҺ�嵹�� AD ��Һ©�� �Ҵ� C �¶ȹ��ߣ��Ҵ��������Ӽ���ˮ�������� 75%
��������
ʵ���������Ҵ���������Ũ���������������ȵ������£���ȡ����������Ȼ������ᴿ���õ�����������������
�� ��ƿ�ڵ�Һ�����ԼΪ3ml+9.5ml+6ml=18.5mol����ƿ��Һ��������������ƿ�ݻ���2/3�Ҳ�����1/3�����50ml���ϡ�
�� ���������л����Ҵ������ᣬ����������ˮ���������������Ȳ����Ҳ�ܲ��������������θ���ܣ����������������⣬���θ�������β��������ݻ��ϴ�Ҳ����ֹ���������á��ʴ�Ϊ����������
�� ����̼������Һ�ܹ��ܽ��Ҵ����к����ᡢ���������ܽ�ȣ��������ķֲ���ŵ�������ζ����ѡAD��
�� �ڢٲ�������Һʱ���еIJ����Ƿ�Һ������������Ϊ����Һ©����
�� �Ȼ��ƿ����Ҵ��γ�CaCl26C2H5OH���ʼ��뱥���Ȼ�����Һϴ�ӣ���Ҫϴȥ�ֲ�Ʒ�е��Ҵ��������������������ͼ��������¶��ܷ���ˮ�⣬��ѡ����ˮ�����ƽ��и��
�� ���������Ϣ����140��ʱ�ᷢ������Ӧ�������ѣ����¶ȹ������������IJ��ʻή�͵Ŀ���ԭ���Ƿ����˸���Ӧ��2CH3CH2OH ![]() CH3CH2OCH2CH3 + H2O��
CH3CH2OCH2CH3 + H2O��
�� �Ҵ������ᷴӦ��������Ϊ��46��60����ʵ�������Ҵ�����Ϊ2.1g����������Ϊ2.4g���Ҵ���������������������ɵ����������������ϼ���õ�����������������CH3COOH��CH3COOC2H5������õ�������������3.52g���õ������IJ�Ʒ����Ϊ2.64g�������������IJ���=2.64g��3.52g��100%=75%��

����Ŀ��һ���¶��£��������ݻ���ͬ�ĺ����ܱ������а���ͬ��ʽͶ�뷴Ӧ�������Ӧ2SO2(g)+ O2(g) ![]() 2SO3(g)(����Ӧ����)����÷�Ӧ������������£�
2SO3(g)(����Ӧ����)����÷�Ӧ������������£�
����1 | ����2 | ����3 | |
��Ӧ�¶�T/K | 700 | 700 | 800 |
��Ӧ��Ͷ���� | 2molSO2��1molO2 | 4molSO3 | 2molSO2��1molO2 |
ƽ��v��(SO2)/mol��L-1��s-1 | v1 | v2 | v3 |
ƽ��c(SO3)/mol��L-1 | c1 | c2 | c3 |
ƽ����ϵ��ѹǿp/Pa | p1 | p2 | p3 |
���ʵ�ƽ��ת����a | ��1 (SO2) | ��2 (SO3) | ��3 (SO2) |
ƽ�ⳣ��K | K1 | K2 | K3 |
����˵����ȷ����
A. v/span>1<v2��c2< 2c1 B. K1>K3��p2> 2p3
C. v1<v3����1(SO2) <��3(SO2) D. c2> 2c3����2(SO3)+��3(SO2)<1
