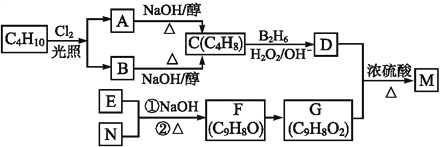
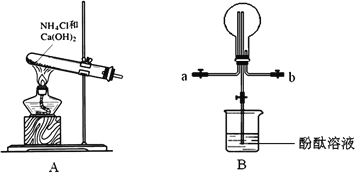
��Ŀ����
����Ŀ��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol��L-1��Na0H ��Һ������ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol��L-1��HA��Һ��BOH��Һ���ζ���������Һ��pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵������ȷ����
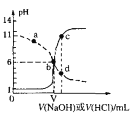
A. HAΪ���ᣬBOHΪǿ��
B. a��ʱ����Һ������Ũ�ȴ��ڹ�ϵ��c(B+)>c(Cl-)>c(OH-)>c(BOH)
C. b��ʱ����ЭҺ��ˮ�ĵ���̶���ͬ����V=20
D. c��d������Һ��Ϻ���֮����ڹ�ϵ��c(H+)= c(OH-)+c(BOH)
���𰸡�D
��������A��Ũ�Ⱦ�Ϊ0.1mol��L-1��HA��Һ��BOH��Һ�����µ�pH�ֱ�Ϊ1��11����֪HAΪǿ�ᣬBOHΪ�����A����B��a������BOH��Һ�еμ�����ϡ���ᣬ��ʱBOH����������ʹ��Һ�Լ��ԣ����ܴ���c(OH-)>c(BOH)����B����C��b��HA��NaOH��Ӧʱ��ҺpH=6�����ɵ��β�ˮ�⣬��V�����ܵ���20����ΪV=20ʱNaA����ҺpH=7����C����D��c��d������Һ��Ϻ�����NaOH��HClǡ����ȫ�кͣ�ֻ����BCl��ˮ�⣬���������غ��֪c(H+)= c(OH-)+c(BOH)����D��ȷ����ΪD��

��ϰ��ϵ�д�
 С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
�����Ŀ