��Ŀ����
�� 4 mol A ����� 2 mol B ������ 2 L �������л�ϲ���һ�������·������·�Ӧ��2A��������B������ 2C������������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ���������м���˵����������ȷ����
2C������������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ���������м���˵����������ȷ����
 2C������������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ���������м���˵����������ȷ����
2C������������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ���������м���˵����������ȷ����| A�������� A ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.3 mol��L��1��s��1 |
| B�������� B ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.6 mol��L��1��s��1 |
| C��2 s ʱ���� A��ת����Ϊ70�� |
| D��2 s ʱ���� B��Ũ��Ϊ 0.7 mol��L��1 |
AD
���� 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ����μӷ�Ӧ��AΪ1.2molBΪ0.6mol����C1.2mol VA="0.3" mol��L��1��s��1 VB=0.15mol��L��1��s��1 A��ȷB����2 s ʱ���� A��ת����1.2/4=0.3��C ����2 s ʱ���� B��Ũ��Ϊ0.7mol��L��1��D��ȷ��

��ϰ��ϵ�д�
�����Ŀ
 2c(g)+D(g)�����ֲ�ͬ�����µķ�Ӧ���ʷֱ�Ϊ
2c(g)+D(g)�����ֲ�ͬ�����µķ�Ӧ���ʷֱ�Ϊ 2SO2+ O2 ��H��0������SO3�ı仯����ͼʾ��
2SO2+ O2 ��H��0������SO3�ı仯����ͼʾ��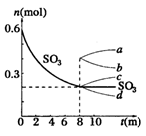
 NH3������ѹǿʹ���������Сʱ����ѧ��Ӧ���ʼӿ죬����Ҫԭ���ǣ� ��
NH3������ѹǿʹ���������Сʱ����ѧ��Ӧ���ʼӿ죬����Ҫԭ���ǣ� �� 2 R(g)��2W(g)��4�ֲ�ͬ����µķ�Ӧ���ʷֱ����£����б�ʾ�÷�Ӧ���������ǣ� ��
2 R(g)��2W(g)��4�ֲ�ͬ����µķ�Ӧ���ʷֱ����£����б�ʾ�÷�Ӧ���������ǣ� ��  2B��g����C��g��������ӦΪ���ȷ�Ӧ����ƽ��ʱ��Ҫʹ����Ӧ���ʽ��ͣ�A��Ũ������Ӧ��ȡ�Ĵ�ʩ�� �� ��
2B��g����C��g��������ӦΪ���ȷ�Ӧ����ƽ��ʱ��Ҫʹ����Ӧ���ʽ��ͣ�A��Ũ������Ӧ��ȡ�Ĵ�ʩ�� �� ��
 ��
��
 mol/L
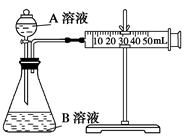
mol/L H2C2O4��Һ����ȡ��֧�Թܸ�����4 mL 0.1 mol/L KMnO4��Һ������֧�Թֳܷ����飨����һ֧ʢ��H2C2O4��Һ��KMnO4��Һ���Թܣ���һ�������ˮ�У���һ�������ˮ�У�����һ��ʱ��ֱ���
H2C2O4��Һ����ȡ��֧�Թܸ�����4 mL 0.1 mol/L KMnO4��Һ������֧�Թֳܷ����飨����һ֧ʢ��H2C2O4��Һ��KMnO4��Һ���Թܣ���һ�������ˮ�У���һ�������ˮ�У�����һ��ʱ��ֱ��� ������¼��Һ��ɫ����ʱ�䡣��ʵ��Ŀ�����о� �Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͬѧʼ��û�п�����Һ��ɫ����ԭ���� ��
������¼��Һ��ɫ����ʱ�䡣��ʵ��Ŀ�����о� �Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͬѧʼ��û�п�����Һ��ɫ����ԭ���� ��