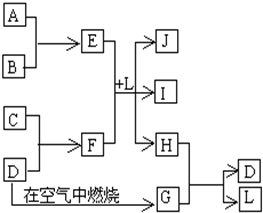
��Ŀ����
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�����Ԫ��A�İ�ɫ��״������IΪNaCl��Һ��D�ǵ���ɫ���嵥�ʡ�����д���пհף�

��1����ͼ���������������ڷǵ���ʵ����ʵĻ�ѧʽ�� ��
��2���õ���ʽ��ʾ��H���γɹ��� ��
��3����E��ˮ��Һ���ɲ����յõ��Ĺ������ʵĻ�ѧΪ ��
��4��F��ˮ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��5��F��ˮ��Һ�Լ��Ե�ԭ�������ӷ���ʽ��ʾ�� ��
��6��E��F��L�з�Ӧ�����ӷ���ʽΪ ��
��7��H��G֮�䷴Ӧ�Ļ�ѧ����ʽΪ .
��1��SO2 ��2��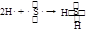 ��3��Al2O3
��3��Al2O3
 ��4��c(Na+)>c(S2-)>c(OH-)>c(HS-)>c(H+) ��5��S2-+H2O
HS-+OH-
��4��c(Na+)>c(S2-)>c(OH-)>c(HS-)>c(H+) ��5��S2-+H2O
HS-+OH-
��6��3Al3++3S2-+6H2O=2Al(OH)3��+3H2S�� ��7��2H2S+SO2=3S��+H2O
��������
���������������Ԫ�صİ�ɫ��״����������������J��������ɫ���嵥����S��GΪSO2������ͼת����֪H�к�����Ԫ�أ�H��SO2����S��L����֪HΪH2S��LΪH2O����E��F��L����Al(OH)3��NaCl��H2S���Ƴ�EΪAlCl3��FΪNa2S��A��B������������C��Na����1�������������ڷǵ���ʵ���SO2����2���õ���ʽ��ʾH2S�γɹ���Ϊ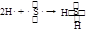 ����3���Ȼ���ˮ�������������������ᣬ�����Ȼ�����Һ�ٽ�ˮ�⣬����ӷ������յ������������壬�������յ���������(4)(5)S2-+H2O
����3���Ȼ���ˮ�������������������ᣬ�����Ȼ�����Һ�ٽ�ˮ�⣬����ӷ������յ������������壬�������յ���������(4)(5)S2-+H2O HS-+OH-��HS-+H2O
HS-+OH-��HS-+H2O H2S+OH-������������Һ��c(Na+)>c(S2-)>c(OH-)>c(HS-)>c(H+)��(6)�Ȼ�����Һ��������Һ����˫ˮ�ⷴӦ�����������������⡣��7����������������������ˮ��
H2S+OH-������������Һ��c(Na+)>c(S2-)>c(OH-)>c(HS-)>c(H+)��(6)�Ȼ�����Һ��������Һ����˫ˮ�ⷴӦ�����������������⡣��7����������������������ˮ��
���㣺���ƶ� �ǵ���� �õ���ʽ��ʾ���ʵ��γɹ��� �����ˮ�� ����Ũ�ȱȽ� ����ʽ����д
��������ɫ��״����һ��������������������ɫ����һ�������������ƣ�Al3+ˮ������ԡ�S2-ˮ��ʼ��ԣ�Al3+��S2-����Һ�з���˫ˮ�ⷴӦ��

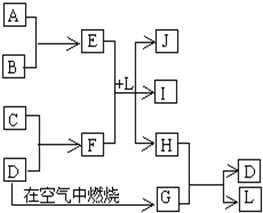 ��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�����Ԫ��A�İ�ɫ��״������IΪNaCl��Һ��D�ǵ���ɫ���嵥�ʣ�����д���пհף�
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�����Ԫ��A�İ�ɫ��״������IΪNaCl��Һ��D�ǵ���ɫ���嵥�ʣ�����д���пհף�
 HS-+OH-
HS-+OH-