��Ŀ����
����Ŀ��ij��ͬѧ������ʵ��̽��Fe2+��Fe3+�����ʡ��ش��������⣺
��1���քeȡһ�����Ȼ������Ȼ����������������Ƴ�0.1mol/L����Һ����FeCl2��Һ�������������м����Ŀ����___________��
��2������ͬѧȡ2mLFeCl2��Һ�����뼸����ˮ���ټ���1��KSCN��Һ����Һ��죬˵��Cl2�ɽ�Fe2+������FeCl2��Һ����ˮ��Ӧ�����ӷ���ʽΪ____________��
��3������ͬѧ��Ϊ�����ʵ�鲻������������ͬѧ��2mLFeCl2��Һ���ȼ���0.5mLú��������Һ�������μ��뼸����ˮ��1��KSCN��Һ����Һ��죬ú�͵�������__________��
��4������ͬѧȡ10mL0.1mol/LKI��Һ������6mL0.1mol/LFeCl3��Һ��ϡ��քeȡ2mL����Һ��3֧����н�������ʵ�飺
����һ֧�Թ��м���1mLCCl4�����������CCl4������ɫ��
���ڶ�֧�Թ��м���1��K3[Fe(CN)6]��Һ��������ɫ������
������֧�Թ��м���1��KSCN��Һ����Һ��졣
ʵ��ڼ����������_______(�����ӷ���)��ʵ��ٺ�ʵ���˵������I-���������������Һ���Ժ���_____(�����ӷ���)���ɴ˿���֤����������ԭ��ӦΪ___________��
��5������ͬѧ��ʢ��H2O2��Һ���Թ��м��뼸���ữ��FeCl2��Һ����Һ����ػ�ɫ��������Ӧ�����ӷ���ʽΪ________��һ��ʱ�������Һ�������ݳ��֣������ȣ�����к��ɫ�������ɣ��������ݵ�ԭ����__________�����ɳ�����ԭ����______________(��ƽ���ƶ�ԭ������)��
��6��ij����������(FexO)1.52g������������������������Һͨ���״����112mLCl2��ǡ�ý�Fe2+��ȫ��������xֵΪ________��
���𰸡� ��ֹFe2+������ 2Fe2++Cl2=2Fe3++2Cl- ��������(�ų�������ʵ���Ӱ��) Fe2+ Fe3+ ���淴Ӧ 2Fe2++H2O2+2H+=2Fe3++2H2O Fe3+��H2O2�ֽ����O2 H2O2�ֽⷴӦ���ȣ��ٽ�Fe3+��ˮ��ƽ�������ƶ� 0.80
�����������������⿼��Fe2+��Fe3+������̽�������ӷ���ʽ����д�����淴Ӧ��̽�����������������ˮ��ƽ���Ӱ��ͻ�ѧ���㡣
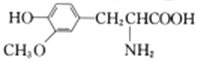
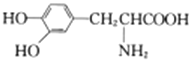
��1��FeCl2�ױ�������O2������������Fe����Fe3+��Ӧ����Fe2+��
��2��FeCl2��Һ����ˮ��Ӧ�����ӷ���ʽΪCl2+2Fe2+=2Fe3++2Cl-��
��3��ú��������ˮ��ú�͵��ܶȱ�ˮС��ú�͵������Ǹ���������
��4��ʵ����������K3[Fe��CN��6]������ɫ������������Fe2+��ʵ����������KSCN��Һ����Һ�������˵����Ӧ�����Һ���Ժ���Fe3+������I-����ʱ��Һ���Ժ�Fe3+���ɴ�˵����������ԭ��ӦΪ���淴Ӧ��
��5��H2O2���ữ��FeCl2��Һ��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��һ��ʱ�����Һ�������ݳ��֣�H2O2��Fe3+���·ֽ����O2�����ɵĺ��ɫFe��OH��3������Fe3+ˮ��ƽ����ƶ����͡�
��6�����ݵ�ʧ�����غ���ʽ��
�������1������FeCl2�ױ�������O2����������������Fe����Fe3+��Ӧ����Fe2+����FeCl2��Һ�м���������м�ɷ�ֹFe2+����������FeCl2��Һ�м���������м��Ŀ���ǣ���ֹFe2+��������
��2����ˮ��FeCl2������FeCl3��FeCl2��Һ����ˮ��Ӧ�����ӷ���ʽΪCl2+2Fe2+=2Fe3++2Cl-��
��3��ú��������ˮ��ú�͵��ܶȱ�ˮС��ú�ͽ�FeCl2��Һ������������ų�O2��ʵ���Ӱ�졣ú�͵������ǣ������������ų�O2��ʵ���Ӱ�죩��
��4������ʵ���١�CCl4������ɫ����˵����Ӧ����I2������ʵ����������K3[Fe��CN��6]������ɫ��������˵����Ӧ����Fe2+������ʵ����������KSCN��Һ����Һ�������˵����Ӧ�����Һ���Ժ���Fe3+��ʵ����������K3[Fe��CN��6]������ɫ������������Fe2+��ʵ������ʵ����˵������I-����������£���Һ���Ժ�Fe3+���ɴ�˵����������ԭ��ӦΪ���淴Ӧ��
��5����ʢ��H2O2��Һ���Թ��м��뼸���ữ��FeCl2��Һ����Һ��Ϊ�ػ�ɫ��˵��Fe2+��H2O2������Fe3+��������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��һ��ʱ�����Һ�������ݳ��֣����ɵ�Fe3+��H2O2�ֽ����O2���������ݵ�ԭ���ǣ�Fe3+��H2O2�ֽ����O2�����ɵĺ��ɫ����ΪFe��OH��3�����ɳ�����ԭ���ǣ�����Һ��Fe3+����ˮ��ƽ����Fe3++3H2O![]() Fe��OH��3+3H+��������ˮ��Ϊ���ȷ�Ӧ��H2O2�ֽ���ȣ��¶����ߣ��ٽ�Fe3+��ˮ��ƽ�������ƶ�����Fe��OH��3������
Fe��OH��3+3H+��������ˮ��Ϊ���ȷ�Ӧ��H2O2�ֽ���ȣ��¶����ߣ��ٽ�Fe3+��ˮ��ƽ�������ƶ�����Fe��OH��3������
��6��n��Cl2��=![]() =0.005mol��FexO��Fe��ƽ����̬Ϊ+
=0.005mol��FexO��Fe��ƽ����̬Ϊ+![]() ��ͨ��Cl2��FeԪ��ȫ��ת��ΪFe3+�����ݵ�ʧ�����غ㣬
��ͨ��Cl2��FeԪ��ȫ��ת��ΪFe3+�����ݵ�ʧ�����غ㣬![]() x
x![]() ��3-
��3-![]() ��=0.005mol
��=0.005mol![]() 2�����x=0.80��
2�����x=0.80��

����Ŀ��һ���¶��£�A��B��C���������о�����ͬһ����Ӧ��N2(g)+3H2(g) ![]() 2NH3(g) ��H =�� Q kJ��mol��1������ͬ��ʱ����ڣ�����������ݣ�
2NH3(g) ��H =�� Q kJ��mol��1������ͬ��ʱ����ڣ�����������ݣ�
���� | A | B | C |
��Ӧ����/mol��L-1��min-1 | v(H2) = 3 | v(N2) = 3 | v(NH3) = 4 |
�����������ų�������Q�Ĵ�С��ϵΪ�� ��
A. B > C > A B. A > B > C C. C > A > B D. B > A > C