��Ŀ����
��14�֣�ij��ɫ��Һ�����п��ܴ����������ӣ�Na����Ag����Ba2����Al3����AlO2�D��S2�D��CO32�D��SO32�D��SO42�D����ȡ����Һ�����й�ʵ�飬�������ͼ��ʾ��
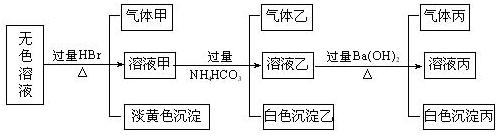
�Իش��������⣺
��1�����ɳ��������ӷ���ʽΪ ��
��2������Һ�����ɳ����ҵ����ӷ���ʽ ��
��3���������� �������һ��ѧʵ����ȷ����ɷ�
��4������ijɷ����ļ��ֿ��� ��
��5���ۺ�������Ϣ�����Կ϶����ڵ������� ��һ�������ڵ������� �����ܴ��ڵ������� ��

�Իش��������⣺
��1�����ɳ��������ӷ���ʽΪ ��
��2������Һ�����ɳ����ҵ����ӷ���ʽ ��
��3���������� �������һ��ѧʵ����ȷ����ɷ�
��4������ijɷ����ļ��ֿ��� ��
��5���ۺ�������Ϣ�����Կ϶����ڵ������� ��һ�������ڵ������� �����ܴ��ڵ������� ��
��14�֣���
��1��2S2�D��SO32�D��6H����3S����3H2O ��1�֣�
��2��Al3����3HCO3�D��Al��OH��3����3CO2����1�֣�
��3��BaCO3��BaCO3��BaSO4�Ļ�����2�֣������ó������м���ϡ���ᣬ������ȫ���ܽ⣬˵����������BaCO3���������������ܽ⣬˵��������BaCO3��BaSO4�Ļ�����2�֣�
��4���������5�ֿ��ܣ���H2S��SO2��CO2��CO2��H2S ��CO2��SO2��2�֣�
��5��Na����S2�D��SO32�D��AlO2�D��2�֣���Ag����Ba2����Al3����2�֣���CO32�D��SO42�D��2�֣�
��1��2S2�D��SO32�D��6H����3S����3H2O ��1�֣�
��2��Al3����3HCO3�D��Al��OH��3����3CO2����1�֣�
��3��BaCO3��BaCO3��BaSO4�Ļ�����2�֣������ó������м���ϡ���ᣬ������ȫ���ܽ⣬˵����������BaCO3���������������ܽ⣬˵��������BaCO3��BaSO4�Ļ�����2�֣�
��4���������5�ֿ��ܣ���H2S��SO2��CO2��CO2��H2S ��CO2��SO2��2�֣�
��5��Na����S2�D��SO32�D��AlO2�D��2�֣���Ag����Ba2����Al3����2�֣���CO32�D��SO42�D��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ